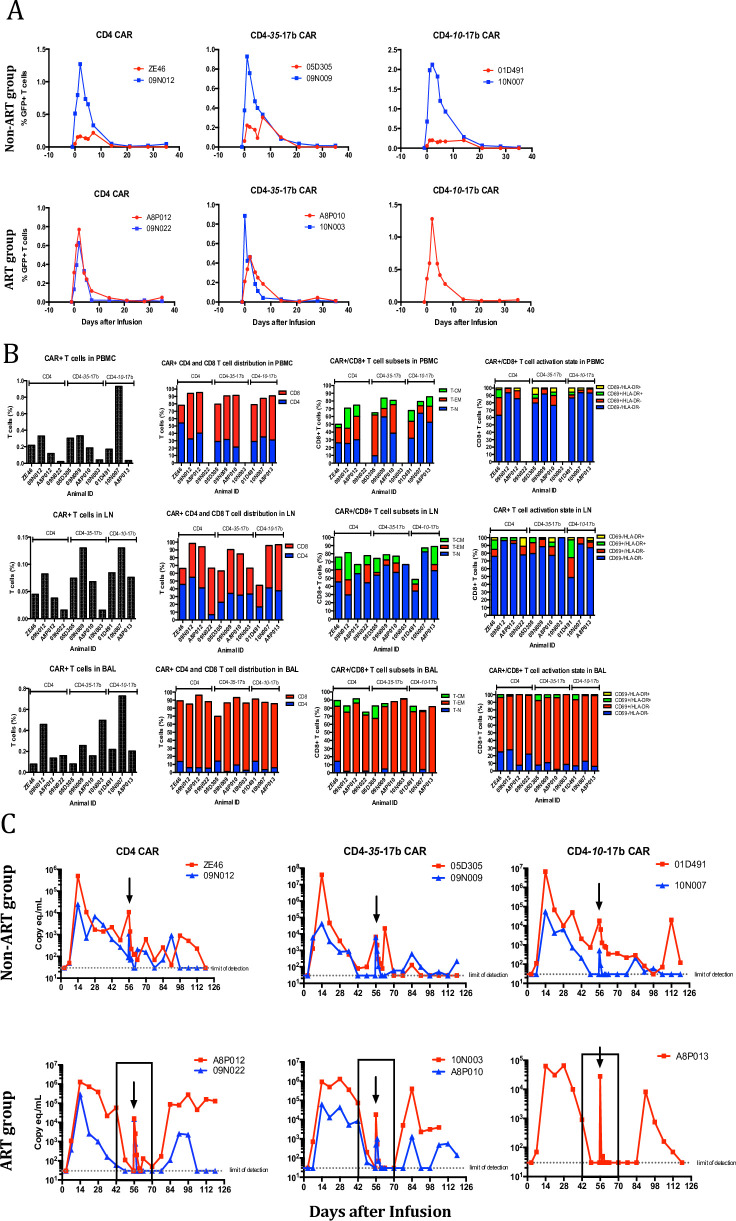
Fig 2. CAR T cell therapy on anti-SHIV-infected animals.
(A) Adoptively transferred CAR-modified T cells were quantified within PBMC via flow-cytometry through the co-expressing fluorescent marker, eGFP, within non-ART treated animals (top row) and ART treated animals (bottom row). CAR+ T cells expand in PBMC within the first two days of adoptive transfer, but rapidly decline and become undetectable after 3 weeks. (B). CAR+ T cells were quantified and immunophenotyped within PBMC (top panel), inguinal lymph node (middle panel) and bronchoalveolar lavage (BAL, bottom panel) samples. Highly activated CD8+/CAR+ T cells traffic to the lung, likely deriving from the expanded cells from the infusion mixture whereas CAR+ cells within PBMC and LN tissue display the same phenotype as unexpanded cells from infusion mixture. *Insufficient CAR+ T cells were obtained for analysis. (C) Plasma viral load measurements indicate a high frequency of animals controlling SHIV infection. Measurements were taken on a weekly basis post-infection to monitor changes in viral loads and to determine if CAR+ T cell infusions exhibited antiviral activity. Arrows indicate time of CAR+ T cell adoptive transfer and the boxed areas in the lower panel designate the time points in which ART groups received therapy. The dramatic increase viral load at the time of infusion is from the transfer of autologous plasma containing SHIV RNA.