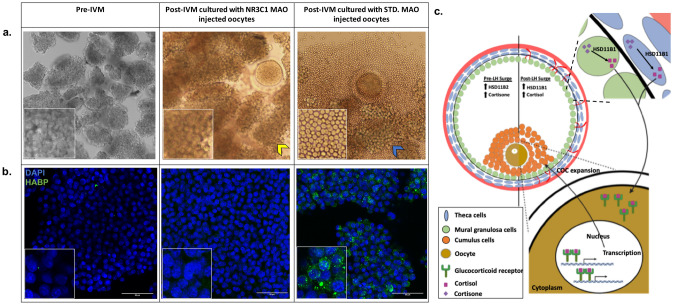
Figure 6.
NR3C1 is essential for cross-communication between the oocyte and the surrounding CCs. (a) Stereomicroscope image of CC morphology immediately after aspiration and prior to IVM (left) and in the presence of a NR3C1 MAO-injected oocyte (middle) versus an STD MAO-injected oocyte (right) after IVM. Note the clumped CCs (image insets) in the pre-IVM and NR3C1 MAO co-cultures (yellow arrowhead) compared to the single CCs visible following expansion (blue arrowhead) (b) IF detection of HA using HABP confirmed a lack of expansion in pre-IVM CCs and those incubated with NR3C1 MAO-injected oocytes as evidenced by the little to no HABP (green) immunostaining in CCs also stained with DAPI (blue). In contrast, robust HABP immunostaining was observed in the CCs co-cultured with STD MAO-injected oocytes. (c) A proposed model for NR3C1-dependent bidirectional communication between the oocyte and somatic cells. The increased relative level of HSD11B1 to HSD11B2 in the mGCs/CCs (green) and the theca cells (blue) convert cortisone to cortisol post-LH surge, with cortisol binding to NR3C1 in the oocyte. Nuclear translocation of cortisol bound NR3C1, activation of transcription, and further unknown downstream events leads to COC expansion that takes place during meiotic maturation of the oocyte.