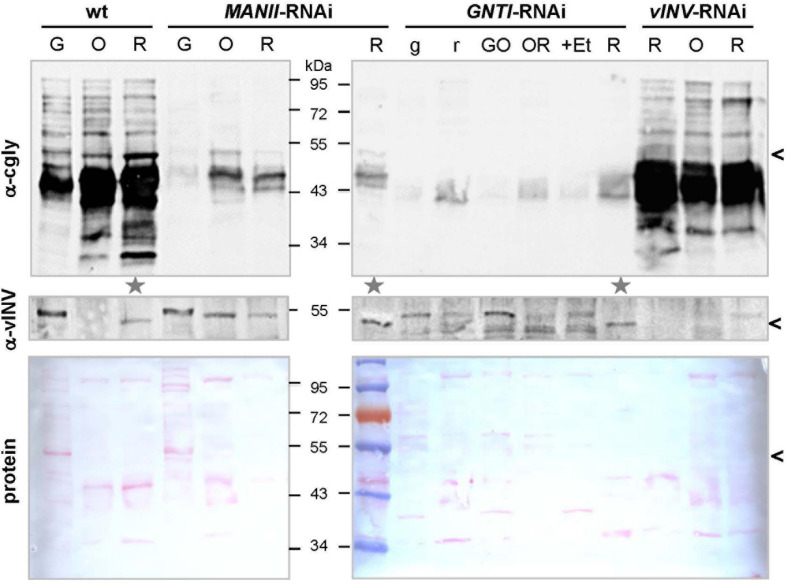
FIGURE 2.
Detection of complex N-glycosylated proteins during tomato fruit ripening. Blots of fruit extracts from Micro-Tom wild-type (wt), MANII-RNAi (#14), GNTI-RNAi (#20), and vInv-RNAi (#9) plants of different ripeness (G, green; O, orange; R, red fruits, with lowercase letters indicating fruit parts; +Et, ethephon treatment) were developed with antisera specific for vacuolar invertase (α-vINV, top) or complex N-glycans (α-cgly, PHA-L, center). The latter mainly recognizes β1,2-xylose and to a lesser extent core α1,3-fucoses (independent antibodies). The Ponceau S-stained blots (protein) are shown as loading reference. Since vacuolar invertase is induced during tomato fruit ripening (migrating ∼50 kDa, avoid in vINV-RNAi), its presence was used as the marker to select fruit extracts of similar ripeness for further use (gray stars). Note that vINV (carrying four complex N-glycans in wild-type) is not the most abundant glycoprotein in red fruits (black arrowheads). Proteins decorated with complex N-glycans accumulate during ripening in wild-type fruits (left), which is markedly reduced in the MANII-RNAi and GNTI-RNAi lines. Apparent molecular masses are indicated in kDa (PageRuler Prestained Protein Ladder, Fermentas).