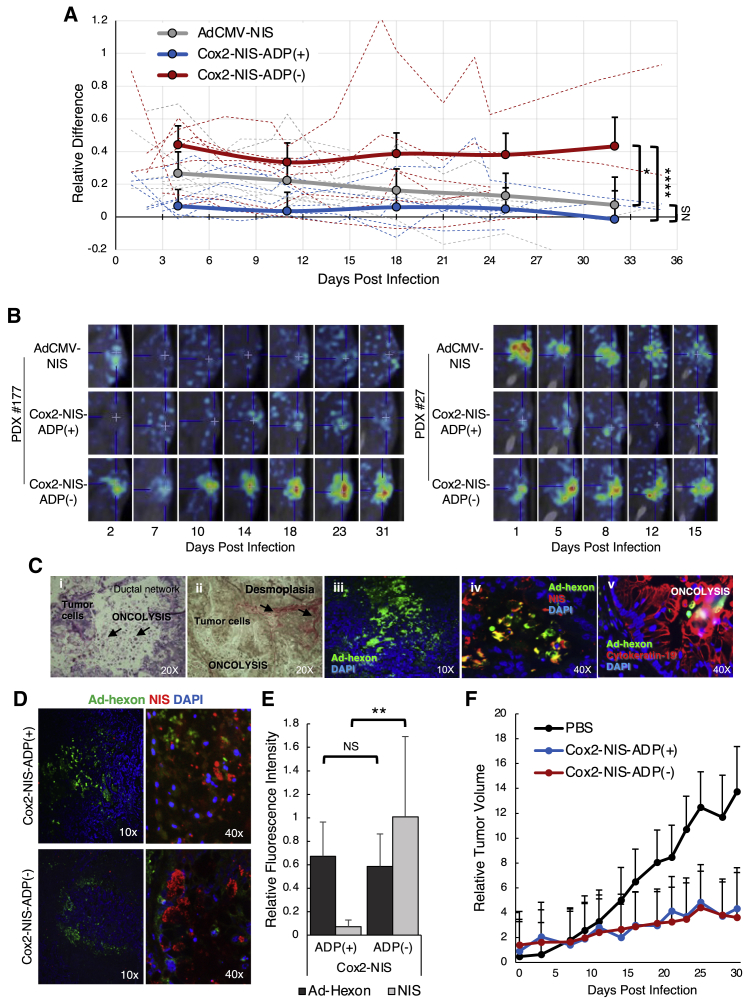
Figure 7.
Radiotracer kinetics and tumor control in PDX
Biopsy-proven PDAC tissues from 6 different human patients were xenografted into groups of mice and injected with 4 × 1010 vp of either Ad-CMV-NIS, Cox2-NIS-ADP(+), Cox2-NIS-ADP(–), or PBS alone, resulting in each patient sample receiving all four treatment conditions. SPECT/CT imaging was performed with 99mTc. (A) Relative difference (RD) in uptake between tumor and muscle was calculated for each animal sample. Average RD of all mice/treatment group graphed in solid lines. Individual mouse RD displayed as dotted lines. Cox2-NIS-ADP(–) infected tumors showed significantly higher levels of radiotracer concentration compared to both Cox2-NIS-ADP(+) (∗p < 0.05; ∗∗∗∗p < 0.0001). (B) Representative images (including final image before mice euthanized) from 2 patient samples showed the improved signal and duration of imaging with Cox2-NIS-ADP(–). (C) Histology and immunofluorescent analyses of sequential slices of PDX tumor infected with Cox2-NIS-ADP(–): (i) H&E staining demonstrating oncolysis within the tumor; (ii) staining for collagen demonstrating desmoplastic nature of pancreatic tumor; (iii) immunostaining for Ad-hexon demonstrating active adenoviral infection in the tumor; (iv) immunostaining for Ad-hexon and NIS demonstrating NIS expression in tumor; (v) immunostaining for Ad-hexon and CK-19, a marker for pancreatic ductal cells demonstrating adenoviral infection and oncolysis in PDAC. (D) PDX tumor immunostaining for Ad-hexon protein and NIS. (E) Quantitative analysis of Ad-hexon and NIS staining using ImageJ software showed improved NIS expression after Cox2-NIS-ADP(–) infection (∗∗p < 0.01; NS, not significant). (F) PDX growth curves. Both Cox2-NIS-ADP(+) and Cox2-NIS-ADP(–) slowed tumor growth compared to control (p = 0.042 and 0.039, respectively) but had no significant difference between the two viruses (p = 0.996). Error bars represent standard deviation.