Figure 6.
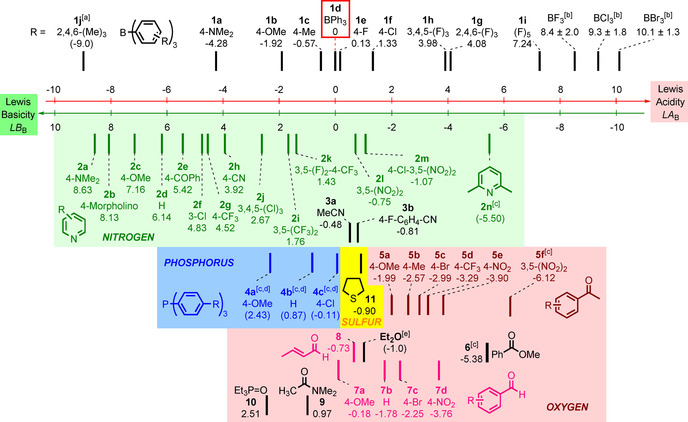
Experimental Lewis acidity and basicity scales for boranes and N‐, O‐, S‐, and P‐centered Lewis bases derived on the basis of Equation (1). Compounds located on the same vertical level combine with an equilibrium constant of K B=1 m −1 in dichloromethane at 20 °C. [a] LA B(1 j) not generally valid. [b] LA B values for BX3 (X=F, Cl, Br) were derived computationally and linked to experiments through isodesmic reactions (see text). [c] Only a single equilibrium constant, K B, was available for the determination of LB B. [d] LB B(4 a–c) are only valid toward Ar3B without ortho substituents (see text). [e] LB B(Et2O) was calculated from data in ref. [19] (see text).
