Table 3.
Activation parameters for enantiomerization of [n]helicenes (n=3 and 6) substituted at the inner, middle, and outer helix.
|
|
Position of methyl group(s) |
ΔH ≠ [kcal mol−1] |
ΔS ≠ [cal K−1 mol−1] |
ΔG ≠ [kcal mol−1] at (T [K]) |
|---|---|---|---|---|
|
|
1 |
38.5 |
−9.8 |
43.0 (542)[a] |
|
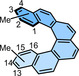
1,14 |
– |
– |
43.1 (543)[a] |
|
|
1,16 |
– |
– |
43.8 (543)[a] |
|
|
1,3,14,16 |
37.7 |
−12.9 |
43.8 (543)[a] |
|
|
2,15 |
– |
– |
40.6 (513)[a] |
|
|
4,13 |
– |
– |
36.3 (469)[a] |
|
|
|
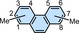
4,5 |
13.9 |
−7.9 |
16.1 (298)[b] |
|
3,4,5,6 |
21.9 |
−3.9 |
23.1 (298)[b] |