Figure 2.
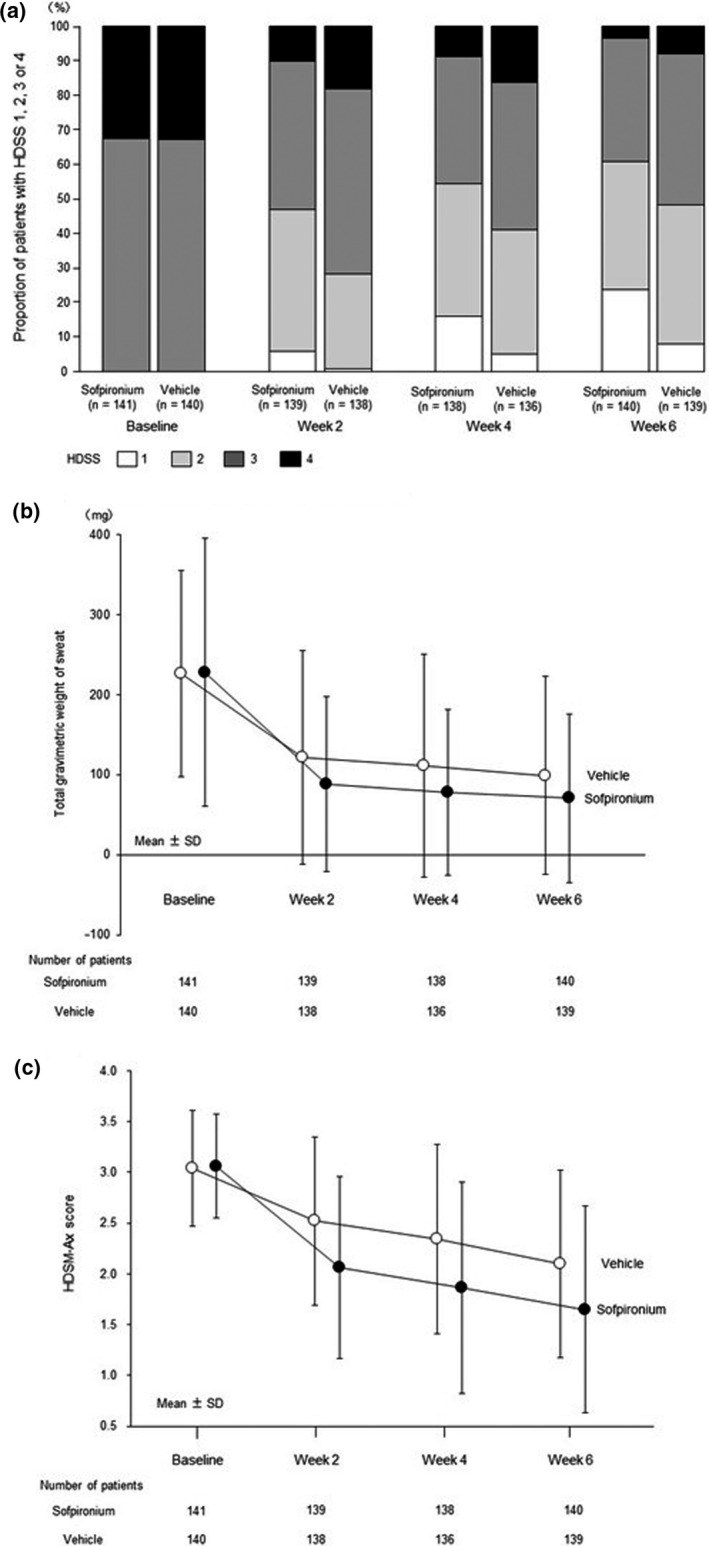
Changes in values of secondary efficacy end‐points at each evaluation point. (a) Changes in proportion of patients with Hyperhidrosis Disease Severity Score (HDSS) of 1, 2, 3 or 4. (b) Changes in total gravimetric weight of sweat. (c) Changes in Hyperhidrosis Disease Severity Measure–Axillary (HDSM‐Ax) score. Data from each patient at baseline and week 6 consisted of the median of measurements at three time points each (baseline‐1 to ‐3 and week 6‐1 to ‐3, respectively), and data from each patient at weeks 2 and 4 consisted of the measurement at one time point each. The patients with no data at each evaluation point were excluded from the analysis.
