Figure 3.
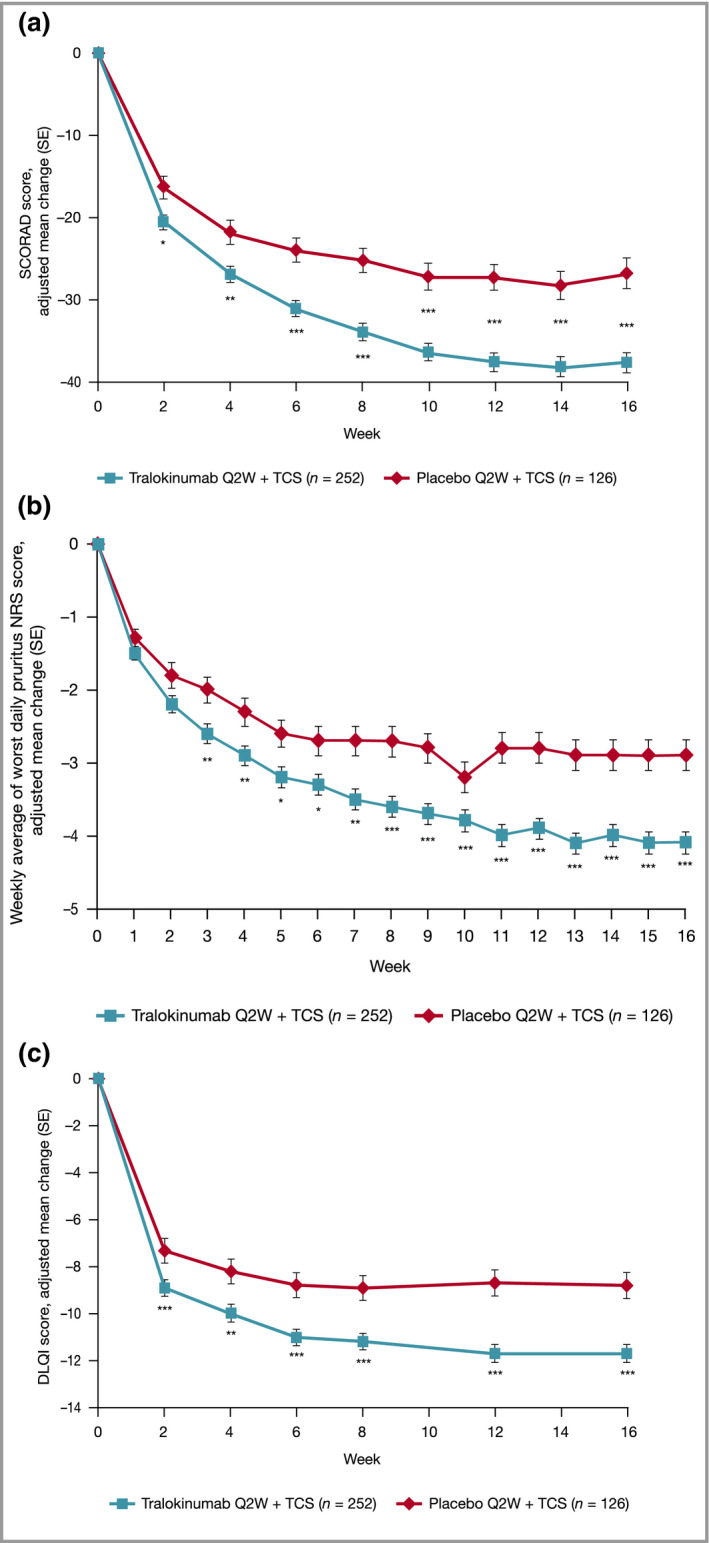
Effect of tralokinumab and placebo treatment on secondary endpoint: (a) change from baseline in SCORAD score by visit; (b) change from baseline in weekly average of worst daily pruritus NRS score; and (c) change from baseline in DLQI score by visit, repeated‐measurements analysis, initial treatment period, full analysis set. Data are adjusted mean change (SE) from repeated‐measurements model. Data collected after permanent discontinuation of investigational medicinal product or initiation of rescue medication not included. The number of patients assessed at each visit can be found in Tables S6–S9; see Supporting Information. In case of no postbaseline assessments before indication of rescue medication, the week 2 (week 1 for NRS) change will be imputed as 0. Repeated‐measurements model: Change = Treatment × Week + Baseline × Week + Region + Baseline Investigator’s Global Assessment. *P < 0·05 vs. placebo + TCS; **P < 0·01 vs. placebo + TCS; ***P < 0·001 vs. placebo + TCS. DLQI, Dermatology Life Quality Index; NRS, Numeric Rating Scale; Q2W, every 2 weeks; SCORAD, SCORing Atopic Dermatitis; SE, standard error; TCS, topical corticosteroids.
