Scheme 1.
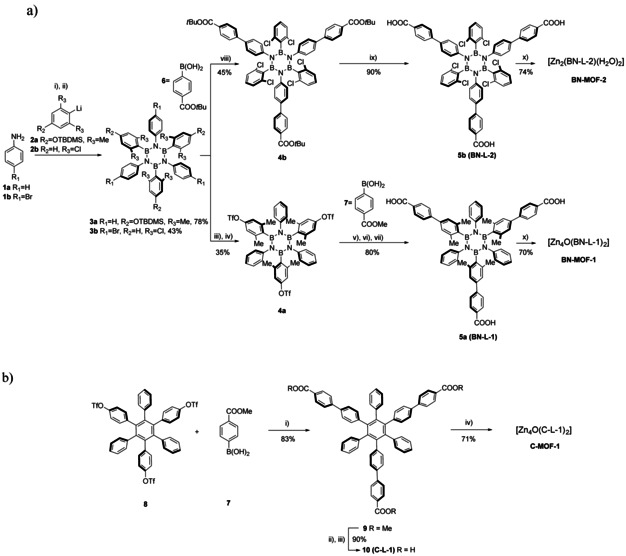
a) Synthesis of three‐carboxyl borazine linkers BN‐l‐1 (5 a) and BN‐L‐2 (5 b), and corresponding BN‐MOF‐1 and BN‐MOF‐2: i) BCl3, toluene, reflux, 18 h; ii) 2, THF, −84 °C to r.t., 24 h; iii) 3 a, TBAF, THF, r.t., 2 h; iv) Tf2O, pyridine, −10 °C, 18 h; v) 7, [Pd(PPh3)4], K2CO3, dioxane, H2O, reflux, 48 h; vi) NaOH (1 m), MeOH, THF, r.t., 18 h; vii) HCl (1 m), r.t., 5 min.; viii) 3 b, [Pd(dba)2], K3PO4, XPhos, THF, 75 °C, 48 h; ix) TFA, CH2Cl2, r.t., 18 h; x) Zn(NO3)2⋅6 H2O, DMF/NMP, 85 °C, 72 h. b) Synthesis of three‐carboxyl full carbon linker C‐L‐1 (10) and C‐MOF‐1. i) [Pd(OAc)2], K3PO4, XPhos, THF, 75 °C, 18 h; ii) NaOH (1 m) MeOH, THF, r.t., 16 h; iii) HCl (1 m), r.t., 5 min; iv) Zn(NO3)2⋅6 H2O, DMF/NMP, 85 °C, 72 h.
