Figure 2.
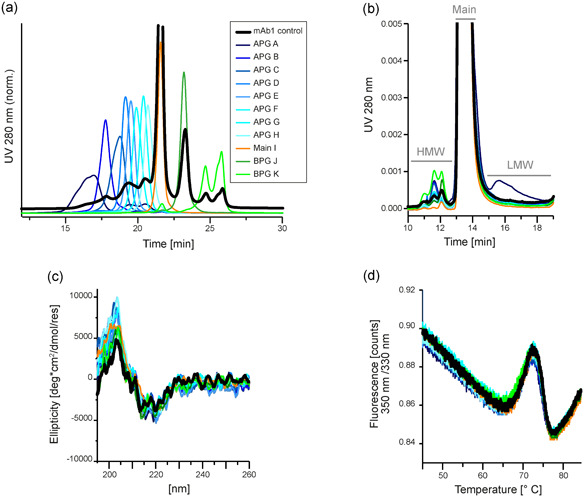
Characterization of weak cation exchange chromatography (WCX)‐fractionated monoclonal antibody 1 (mAb1) indicates biophysical stability of mAb1 charge variants. mAb1 was separated by cation exchange chromatography and individual charge fractions were collected and characterized. The fractions include species from the acidic peak group A–H, the main species I, and species from the basic peak group J–K. As a control, fractions were pooled again to result in a sample including all charge variants, which was subjected to the same conditions as the fractions. (a) Purity of the collected fractions was verified by reinjection of the samples onto the WCX column. The fractions were thus suitable for further characterization. (b) Separation of the fractions by analytical size‐exclusion chromatography (SEC) revealed the size distribution within the charge variants. All charged species contain monomeric mAb1. The level of high molecular weight (HMW) species were enhanced in more basic fractions, and low molecular weight (LMW) species are found only in acidic fractions. Full data of the SEC measurements is shown in Table S1. (c) Circular dichroism spectroscopy showed no differences between the collected fractions, and thus indicated structural integrity of all mAb1 charge variants. (d) Nano differential scanning fluorimetry (nano‐DSF) measurements of the collected fractions resulted in highly comparable profiles and melting temperatures, verifying that protein stability is sustained in mAb1 charge variants. Full data of the nano‐DSF measurements is shown in Table S1 [Color figure can be viewed at wileyonlinelibrary.com]
