Figure 1:
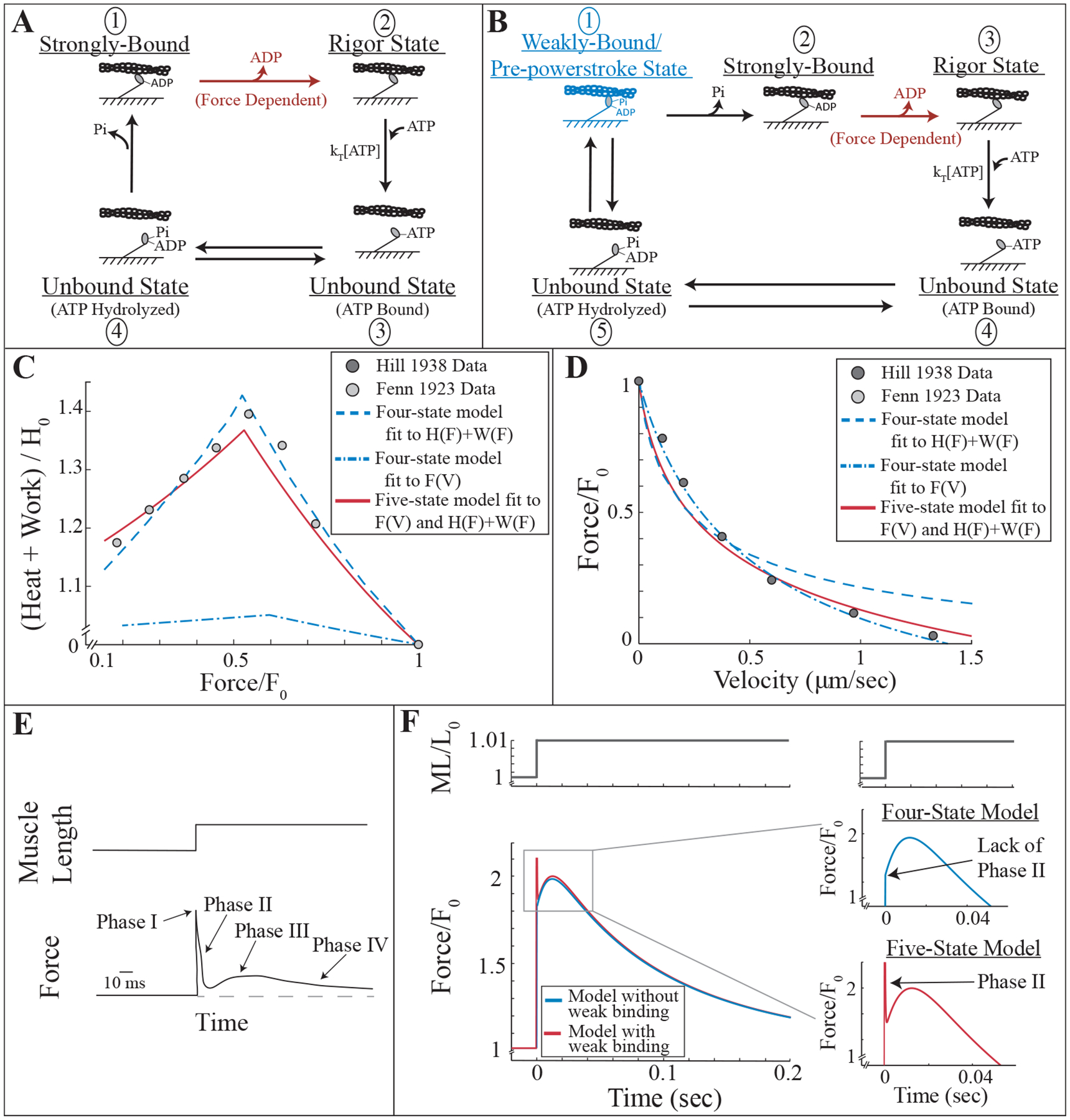
Motivation for weakly-bound cross-bridges in a model of muscle contraction based on molecular measurements. A) Four-state kinetic scheme of myosin’s interaction with actin including two bound states (states 1 and 2) and two unbound states (states 3 and 4). B) Five-state kinetic scheme that is the same as that in A, except with the addition of a weakly-bound state labeled in blue (state 1), where myosin is bound to actin but has not undergone its power-stroke. C, D) Measurements of the heat produced and work done by a muscle as a function of load (C, [18]) and force produced as a function of shortening velocity (D, [16]). The four-state model was fit to the energetics data (dashed blue line in C), and the corresponding force-velocity relationship is plotted in D. The four-state model was also fit to the force-velocity measurements (dash-dotted blue line in D), and the corresponding energetics relationship is plotted in C. The five-state model is able to simultaneously fit both measurements (red lines, C and D). E) Illustration of a typical skeletal muscle force transient response to a quick stretch. F) Four- and five-state model response to quick stretch (blue and red lines, respectively). Insets illustrate the lack of phase II in the four-state model response, and the clear phase II in the five-state model response.
