Figure 4.
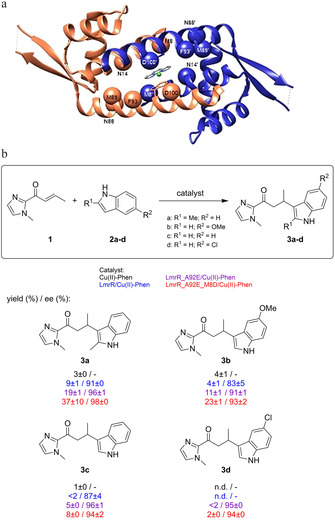
a) Structure of LmrR/CuII‐Phen with residues that were randomized during the directed evolution study indicated as spheres. b) Scope of the enantioselective Friedel–Crafts alkylation catalyzed by CuII‐Phen and CuII‐Phen/LmrR artificial metalloenzyme mutants. Conditions: 12 μM LmrR mutant, 9 μM CuII‐Phen, 1 mM 1 and 2 a in 20 mM MOPS, 150 mM NaCl, pH 7 at 4 °C. Reaction times: 30 min (3 a), 6 h (3 b, 3 c) or 24 h (3 d). Values are given as the average of independent duplicate experiments, each performed in duplo. Errors are given as standard deviations.
