Figure 4.
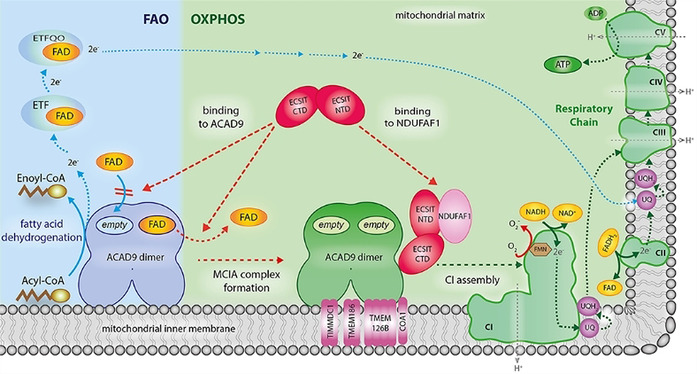
Proposed model of how deflavination of ACAD9 by ECSIT permits the coordinated regulation of the FAO and OXPHOS pathways. Clockwise from lower left: In the absence of ECSIT, ACAD9 acts as an acyl‐CoA dehydrogenase enzyme in the first step of the fatty acid β‐oxidation (FAO) pathway. To exert this function, ACAD9 needs the insertion of FAD prosthetic group into the catalytic pocket. Then, dehydrogenation of the acyl‐CoA substrate into an enoyl‐CoA intermediate metabolite is concomitant with the reduction of the FAD into FADH2 (solid blue lines). This allows the transfer of electrons to the FAD bound to electron transfer flavoprotein (ETF) and subsequently to the ETF‐ubiquinone oxireductase (ETF‐QO), ultimately allowing the electrons to reach the ubiquinone (UQ) pool in the respiratory chain (dashed blue lines). Lower left to lower right: ECSIT binding induces the ejection of FAD from ACAD9 and also inhibits the FAD reuptake step (dashed red lines), shutting down its dehydrogenase activity. This antagonizes the function of ACAD9 in FAO and results in its redeployment as a CI assembly factor. In parallel, the ability of ECSIT to recruit NDUFAF1 (dashed red line) allows formation of the entire MCIA holocomplex with the other membrane components TIMMDC1, TMEM186, TMEM126B and COA1. The latter is required for the assembly of a functional CI and subsequently for the activation of the respiratory chain as part of the OXPHOS system, implying the pumping of a proton gradient (H+, grey dashed lines) across the mitochondrial inner membrane that ultimately drives the generation of ATP (green dashed lines). For sake of clarity, only the binding of NDUFAF1‐ECSIT to one of the ACAD9 monomers is shown.
