Abstract
Protocols for the synthesis of the bulky polyfluorinated triarylboranes 2,6‐(C6F5)2C6F3B(C6F5)2 (1), 2,6‐(C6F5)2C6F3B[3,5‐(CF3)2C6H3] (2), 2,4,6‐(C6F5)3C6H2B(C6F5)2 (3), 2,4,6‐(C6F5)3C6H2B[3,5‐(CF3)2C6H3] (4) were developed. All boranes are water tolerant and according to the Gutmann‐Beckett method, 1–3 display Lewis acidities larger than that of the prominent B(C6F5)3.
Keywords: fluorinated compounds, kinetic stabilization, Lewis acids, terphenyl, water tolerant boranes
Water tolerant boranes: The synthesis and characterization of bulky polyfluorinated triarylboranes 2,6‐(C6F5)2C6F3B(C6F5)2 (1), 2,6‐(C6F5)2C6F3B[3,5‐(CF3)2C6H3] (2), 2,4,6‐(C6F5)3C6H2B(C6F5)2 (3), 2,4,6‐(C6F5)3C6H2B[3,5‐(CF3)2C6H3] (4) is presented. Unlike the prominent B(C6F5) („BCF“), 1–4 are water tolerant and 1–3 even show larger Lewis acidities, as determined by the Gutmann‐Beckett method.
Tris(pentafluorophenyl)borane, B(C6F5)3 (i, „BCF“) is arguably one of the most versatile Lewis acids, which has found numerous applications in organic and organometallic chemistry, catalysis and materials science (Scheme 1).[ 1 , 2 , 3 , 4 , 5 , 6 ]
Scheme 1.

Polyfluorinated triarylboranes.
A prominent application involves the use in frustrated Lewis pair (FLP) chemistry for the activation of small molecules. The mixture of B(C6F5)3 (i) with tBu3P or Mes3P comprises FLPs, that amongst others,[ 7 , 8 , 9 , 10 , 11 ] are capable to activate dihydrogen. [12] The frustration of these FLPs relies on the steric encumbrance of tBu3P and Mes3P, in contrast to the fact that B(C6F5)3 (i) and the smaller Ph3P react to give a regular, inactive Lewis pair Ph3PB(C6F5)3. [13] The high Lewis acidity of B(C6F5)3 stems from the large number of fluorine atoms that decorate the substituents, which give rise to a high charge separation and provide delocalization of the negative charge.[ 14 , 15 ] Tris(3,4,5‐trifluorophenyl)borane, B(3,4,5‐F3C6H2)3 (ii) and tris[3,5‐bis(trifluoromethyl)phenyl]borane, B[3,5‐(CF3)2C6H3]3 (iii, „BArF“) are alternative, less frequently used Lewis acids (Scheme 1), which are slightly less acidic, but possess slightly more accessible boron atoms than BCF (i).[ 16 , 17 , 18 , 19 ] This is an advantage for some applications in catalysis, such as hydroboration reactions. BArF (iii) forms a FLP with the bulky 2,2,6,6‐tetramethylpiperidine, which is also capable of activating dihydrogen. The frustration is again due to the steric encumbrance of the Lewis base. [20] Tris[2,4‐bis(trifluoromethyl)phenyl]borane, B[2,4‐(CF3)2C6H3]3 (iv) and tris[2,5‐bis(trifluoromethyl)phenyl]borane, B[2,5‐(CF3)2C6H3]3 (v), the structural isomers of BArF (iii), are less active Lewis acids due to the steric shielding, and more importantly, the presence of intramolecular B⋅⋅⋅F interactions (Scheme 1),[ 21 , 22 ] which are absent in the structure of BArF (iii). [23] Recently, tris(perfluorotolyl)borane B[4‐(CF3)C6F4]3 (vi, „BTolF“) was introduced as another alternative to BCF (i), which comprises the highest Lewis acidity amongst these polyfluorinated triarylboranes (Scheme 1).[ 24 , 25 ] In fact, the fluorine ion affinity (FIA), one of the criteria to evaluate the Lewis acidity, slightly exceeds that of the benchmark SbF5 (493 kJ mol−1), which qualifies BTolF (vi, 499 kJ mol−1) as a Lewis super acid. [26] One of the major drawbacks of these polyfluorinated boranes is their inherent affinity to (trace amounts of) water. For instance, BCF (i) is known to form isolable water adducts like (C6F5)3BOH2⋅L (L=nil, C6H5, tBuOH, 2H2O) and related hydroxo‐anions [(C6F5)3BOH]−, [(C6F5)3B(μ‐OH)B(C6F5)3]− and [(C6F5)3B(OH)(H2O)B(C6F5)3]− under ambient conditions.[ 27 , 28 , 29 , 30 , 31 , 32 , 33 ] Attempts to dehydrate these water adducts at elevated temperatures are impaired by hydrolytic B−C bond cleavage and give rise to [(C6F5)2BOH]3 and C6F5H. [34] Nonetheless, Lewis acid catalyzed reactions under non‐inert conditions have been demonstrated for chlorinated arylboranes. [35]
While the known portfolio of polyfluorinated triarylboranes allows some fine‐tuning of their Lewis acidity and the accessible space around the boron atoms, neither of these Lewis acids is sterically encumbered enough to form FLPs with smaller Lewis bases, which motivated us to develop synthetic routes for a set four bulky polyfluorinated m‐terphenyldiarylboranes.
The reaction of the lithium reagents 2,6‐(C6F5)2C6F3Li [36] or 2,4,6‐(C6F5)3C6H2Li [37] with the diarylchloroboranes (C6F5)2BCl [38] and [3,5‐(CF3)2C6H3]2BCl,[ 39 , 40 ] respectively, provided the four polyfluorinated triarylboranes 2,6‐(C6F5)2C6F3B(C6F5)2 (1), 2,6‐(C6F5)2C6F3B[3,5‐(CF3)2C6H3] (2), 2,4,6‐(C6F5)3C6H2B(C6F5)2 (3), 2,4,6‐(C6F5)3C6H2B[3,5‐(CF3)2C6H3] (4) in good yields varying between 50 % and 67 % (Scheme 2).
Scheme 2.

Synthesis of bulky polyfluorinated triarylboranes 1–4.
Compounds 1–4 are characterized by very broad 11B NMR resonance signals at δ (CD2Cl2) 60.5 ppm (1), 68.3 ppm (2), 63.1 ppm (3), and 67.9 ppm (4) in the same range as other fluorinated triarylboranes (59.1 ppm [40] for i, 68.1 ppm [20] for ii). The 19F NMR spectra of 1–4 display somewhat broad, mostly featureless resonance signals corresponding to the C6F5 aryl groups attached to boron or in ortho position on the terphenyl substituent. Despite this detail, the 19F NMR spectra indicate that the molecular structures of 1–4 are symmetrical in solution and retain a significant of degree of fluxionality, the latter being of importance in modulating the interaction between the Lewis acidic boron centre and nucleophiles.
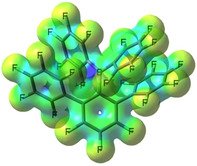
The molecular structures of 1–4 (Figure 1) [41] reveal that the boron atoms adopt distorted trigonal bipyramidal arrangements defined by a C3F2 donor set, with all C−B bonds disposed co‐planar and the interacting F atoms in axial positions. All structures feature a set of two F⋅⋅⋅B interactions between the F1 and F1i/F6, two fluorine atoms located in ortho position on the flanking C6F5 groups of the terphenyl substituent, and boron. Compounds 1 (F1⋅⋅⋅B1 2.681(2) Å), and 3 (F1⋅⋅⋅B1 2.907(3) Å, F6⋅⋅⋅B1 2.722(3) Å) which have two C6F5 rings bonded to boron have two relatively short contacts, while 2 (F1⋅⋅⋅B1 3.040(2) Å, F6⋅⋅⋅B1 3.132(2) Å), and 4 (F1⋅⋅⋅B1 3.247(2) Å, F6⋅⋅⋅B1 2.906(2) Å), each having two 3,5‐(CF3)2C6H3 rings attached to boron, display larger F⋅⋅⋅B contact distances. From this it becomes immediately obvious that the two very electron deficient C6F5 groups increase the Lewis acidity at boron significantly more than the two 3,5‐(CF3)2C6H3 aryl groups, while the impact of the terphenyl used is more subtle, 2,6‐(C6F5)2C6F3 appearing to have a slightly more electron withdrawing effect on boron compared with 2,4,6‐(C6F5)3C6H3.
Figure 1.

Molecular structures of 1–4 and 1⋅H2O⋅2(CH3)2CO showing 30 % probability ellipsoids and the essential atom numbering scheme, and projection of the calculated electrostatic potential onto a 0.025 ē⋅bohr−3 isodensity surface for the gas phase optimized geometries of 1–4 (M06‐2X/6–311++G** level of theory). For clarity, all hydrogen atoms except for those of the H2O molecule coordinated to boron in compound 1⋅H2O⋅2(CH3)2CO were omitted. Symmetry equivalent atoms are designated with superscript i (1−x, y, 0.5−z). Selected bond parameters [Å, °] for 1: C1−B1 1.569(5), C11−B1 1.576(4), F1⋅⋅⋅B1 2.681(2), C1−B1−C11 120.2(2), C11−B1−C11i 119.7(3); for 2: C1−B1 1.576(3), C19−B1 1.564(2), C27−B1 1.562(2), F1⋅⋅⋅B1 3.040(2), F6⋅⋅⋅B1 3.132(2), C1−B1−C19 121.3(1), C1−B1−C27 118.8(1), C19−B1−C27 119.9(2); for 3: C1−B1 1.573(2), C25−B1 1.578(3), C31−B1 1.573(3), F1⋅⋅⋅B1 2.907(3), F6⋅⋅⋅B1 2.722(3), C1−B1−C25 120.3(2), C1−B1−C31 120.5(2), C25−B1−C31 119.2(2); For 4: C1−B1 1.575(2), C25−B1 1.575(2), C25−B1 1.567(2), C33−B1 1.559(2), F1⋅⋅⋅B1 3.247(2), F6⋅⋅⋅B1 2.909(2), C1−B1−C25 119.3(1), C1−B1−C33 121.3(1), C25‐B1−C33 119.4(1); for 1⋅H2O⋅2(CH3)2CO: O1−B1 1.588(2), C1−B1 1.645(2), C19−B11.637(2), C25−B1 1.645(2), O1−B1−C1 110.0(1), O1−B1−C19 103.6(1), O1−B1−C25 102.5(1), C1−B1−C19 113.3(1), C1−B1−C15 109.7(1), C19−B1−C25 116.9(1), O1−H1A 0.90(2), O1−O3 2.625(1), O1−H1B 0.92(2) O1−O2 2.612(1).
The acceptor numbers determined according to the Gutmann‐Beckett method (using Et3PO in CD2Cl2) indicate that 1 (AN 87) is the most Lewis acidic borane in the series, while 2, and 3 (AN 85) are only slightly less acidic. Thus 1–3 display Lewis acidities between those of BF3 (AN 89 [42] ) and B(C6F5)3 (AN 82 [15] ). It was not possible to determine the acceptor number of 4 as the 31P NMR spectrum indicated two very broad signals at room temperature and a number of resonance signals at low temperature (see Supporting Information), indicating a dynamic process. However, from the fact that the chemical shifts of the two broad resonances at room temperature (ca. 76, and 57 ppm) are at lower frequencies than in the case of complexes of 1–3 with Et3PO, we can speculate that the acceptor number of 4 and its Lewis acidity are the smallest in this series.
Unlike the previously known polyfluorinated boranes, such as BCF (i),[ 27 , 28 , 29 , 30 , 31 , 32 , 33 ] 1–4 are air and water tolerant in solid state and can be safely stored in air for at least several weeks. The boranes 1–4 were isolated free of water after work‐up protocols of the crude reaction mixtures in open atmosphere that even involved the washing of the organic layers (CH2Cl2, toluene) with water. However, a subtle difference was observed for 1 and 3 when acetone was used as solvent. In this case, hydrates are indeed formed. The molecular structure of 1⋅H2O⋅2(CH3)2CO (Figure 1) shows that the boron atom adopts a distorted tetrahedral C3O coordination environment. Two acetone molecules are involved in hydrogen bonding with the water molecule coordinated to boron. The O−B bond length (1.588(2) Å) is only marginally longer than that of (C6F5)3BOH2⋅2 H2O (1.577(1) Å). [27] Although NMR spectroscopy indicated the formation of a presumably similar hydrate of 3 (see Supporting Information), crystals suitable for the determination of its molecular structure by X‐ray diffraction were not obtained. The dehydration of these water adducts was easily achieved at reduced pressure (room temperature). For compounds 2 and 4 formation of hydrates was not observed in acetone or other solvents.
In order to evaluate the Lewis acidity of 1–4, i–vi and reference Lewis acids SbF5 and AlCl3, fluoride ion affinity (FIA) and hydride ion affinity (HIA) values have been computed at MP2/6–311++G(2d,2p)//M06‐2X/6–311++G** level of theory, using isodesmic reactions Scheme (Figure 2). [43]
Figure 2.

FIA and HIA values (in kJ mol−1) of 1–4 and related Lewis acids. MP2/6–311++(2d,2p)//M06‐2X/6‐311++G** level of theory. Comparative literature values are given in parentheses.
Both FIA and HIA values are slightly larger than literature values,[ 44 , 45 ] but the difference does not exceed 10 kJ mol−1. According to the computed FIA and HIA values, the Lewis acidity decreases in the order 2>4>1>3, which somewhat differs from the results of the Gutmann‐Beckett method. Overall, these values lie between those of the previously known polyfluorinated triarylboranes i–vi, which is potentially useful if fine‐tuning of the Lewis acidity is needed. In more detail, the bulky 2,4,6‐(C6F5)3C6H2 substituent lowers FIA by 22 kJ mol−1 with respect to C6F5 group; substitution of C6F5 by 2,6‐(C6F5)2C6F3 group lowers FIA by 10 kJ mol−1, while substitution of C6F5 by 3,5‐(CF3)2C6H3 group increases FIA by 13 kJ mol−1. Thus, the largest cumulative effect is achieved in 2, which is the strongest Lewis acids amongst 1–4. The molecular electrostatic potentials of 1–4 show that the Lewis acidic boron atoms are effectively shielded from the direct attack of nucleophiles by bulky fluorinated substituents.
In summary, a series of bulky polyfluorinated triarylboranes 2,6‐(C6F5)2C6F3B(C6F5)2 (1), 2,6‐(C6F5)2C6F3B[3,5‐(CF3)2C6H3] (2), 2,4,6‐(C6F5)3C6H2B(C6F5)2 (3), 2,4,6‐(C6F5)3C6H2B[3,5‐(CF3)2C6H3] (4) has been prepared and fully characterized. Our calculations indicate that 1–4 are competent Lewis acids with FIA and HIA values that compete with (and in some cases are higher than) some of the classic boranes, such as B(C6F5)3 (i, „BCF“) and B[3,5‐(CF3)2C6H3]3 (iii, „BArF“). Unlike the latter, 1–4 are essentially air‐stable and water‐tolerant. The major difference between 1–4 and previous known fluorinated boranes i–vi, however, is the steric encumbrance of the boron atoms that is brought by the bulky m‐terphenyl substituents. We expect that these properties will be very useful for applications in frustrated Lewis pair (FLP) chemistry. We envisage FLP combinations of bulky 1–4 and less bulky Lewis bases, such as Cy3P or Ph3P, which give regular, inactive Lewis pairs with the previously known polyfluorinated triarylboranes i–vi. We are currently investigating such FLPs for the activation of small molecules.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
The Deutsche Forschungsgemeinschaft (DFG) and Romanian National Authority for Scientific Research and Innovation, CNCS‐UEFISCDI, project number PN‐II‐RU‐TE‐2014‐4‐0906, are gratefully acknowledged for financial support. C. S. acknowledges Erasmus+ for a training scholarship. Computational work was financially supported by the Russian Science Foundation (grant no. 18‐13‐00196 to A.Y.T.). Use of equipment of Resource center „Computing Center“ of the Scientific park of St. Petersburg State University is gratefully acknowledged. Open access funding enabled and organized by Projekt DEAL.
C. Stoian, M. Olaru, T. A. Cucuiet, K. T. Kegyes, A. Sava, A. Y. Timoshkin, C. I. Raţ, J. Beckmann, Chem. Eur. J. 2021, 27, 4327.
Dedicated to Professor Cristian Silvestru on the occasion of his 65 th birthday
Contributor Information
Prof. Dr. Alexey Y. Timoshkin, Email: a.y.timoshkin@spbu.ru.
Prof. Dr. Ciprian I. Raţ, Email: ciprian.rat@ubbcluj.ro.
Prof. Dr. Jens Beckmann, Email: j.beckmann@uni-bremen.de.
References
- 1. Piers W. E., Chivers T., Chem. Soc. Rev. 1997, 26, 345–354. [Google Scholar]
- 2. Chivers T., J. Fluorine Chem. 2002, 115, 1–8. [Google Scholar]
- 3. Erker G., Dalton Trans. 2005, 1883–1890. [DOI] [PubMed] [Google Scholar]
- 4. Piers W. E., Marwitz A. J. V., Mercier L. G., Inorg. Chem. 2011, 50, 12252–12262. [DOI] [PubMed] [Google Scholar]
- 5. Melen R. L., Chem. Commun. 2014, 50, 1161–1174. [DOI] [PubMed] [Google Scholar]
- 6. Lawson J. R., Melen R. L., Inorg. Chem. 2017, 56, 8627–8643. [DOI] [PubMed] [Google Scholar]
- 7. Stephan D. W., Erker G., Angew. Chem. Int. Ed. 2010, 49, 46–76; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 50–81. [Google Scholar]
- 8. Stephan D. W., J. Am. Chem. Soc. 2015, 137, 10018–10032. [DOI] [PubMed] [Google Scholar]
- 9. Stephan D. W., Acc. Chem. Res. 2015, 48, 306–316. [DOI] [PubMed] [Google Scholar]
- 10. Stephan D. W., Erker G., Angew. Chem. Int. Ed. 2015, 54, 6400–6441; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2015, 127, 6498–6541. [Google Scholar]
- 11. Melen R. L., Angew. Chem. Int. Ed. 2018, 57, 880–882; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 890–892. [Google Scholar]
- 12. Welch G. C., Stephan D. W., J. Am. Chem. Soc. 2007, 129, 1880–1881. [DOI] [PubMed] [Google Scholar]
- 13. Jacobsen H., Berke H., Döring S., Kehr G., Erker G., Fröhlich R., Meyer O., Organometallics 1999, 18, 1724–1735. [Google Scholar]
- 14. Morgan M. M., Marwitz A. J. V., Piers W. E., Parvez M., Organometallics 2013, 32, 317–322. [Google Scholar]
- 15. Sivaev I. B., Bregadze V. I., Coord. Chem. Rev. 2014, 270–271, 75–88. [Google Scholar]
- 16. Yin Q., Kemper S., Klare H. F. T., Oestreich M., Chem. Eur. J. 2016, 22, 13840–13844. [DOI] [PubMed] [Google Scholar]
- 17. Yin Q., Soltani Y., Melen R. L., Oestreich M., Organometallics 2017, 36, 2381–2384. [Google Scholar]
- 18. Seo Y., Lowe J. M., Gagné M. R., ACS Catal. 2019, 9, 6648–6652. [Google Scholar]
- 19. Carden J. L., Gierlichs L. J., Wass D. F., Browne D. L., Melen R. L., Chem. Commun. 2019, 55, 318–321. [DOI] [PubMed] [Google Scholar]
- 20. Herrington T. J., Thom A. J. W., White A. J. P., Ashely A. E., Dalton Trans. 2012, 41, 9019–9022. [DOI] [PubMed] [Google Scholar]
- 21. Cornet S. M., Dillon K. B., Entwistle C. D., Fox M. A., Goeta A. E., Goodwin H. P., Marder T. B., Thompson A. L., Dalton Trans. 2003, 4395–4405. [Google Scholar]
- 22. Blagg R. J., Lawrence E. J., Resner K., Oganesyan V. S., Herrington T. J., Ashley A. E., Wildgoose G. G., Dalton Trans. 2016, 45, 6023–6031. [DOI] [PubMed] [Google Scholar]
- 23. Konze W. V., Scott B. L., Kubas G. J., Chem. Commun. 1999, 1807–1808. [Google Scholar]
- 24. Nagase M., Kuninobu Y., Kanai M., J. Am. Chem. Soc. 2016, 138, 6103–6106. [DOI] [PubMed] [Google Scholar]
- 25. Körte L. A., Schwabedissen J., Soffner M., Blomeyer S., Reuter C. G., Vishnevskiy Y. V., Neumann B., Stammler H.-G., Mitzel N. W., Angew. Chem. Int. Ed. 2017, 56, 8578–8582; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 8701–8705. [Google Scholar]
- 26. Greb L., Chem. Eur. J. 2018, 24, 17881–17896. [DOI] [PubMed] [Google Scholar]
- 27. Danopoulos A. A., Galsworthy J. R., Green M. L. H., Cafferkey S., Doerrer L. H., Hursthouse M. B., Chem. Commun. 1998, 2529–2530. [Google Scholar]
- 28. Drewitt M. J., Niedermann M., Baird M. C., Inorg. Chim. Acta 2002, 340, 207–210. [Google Scholar]
- 29. Doerrer L. H., Green M. L. H., Dalton Trans. 1999, 4325–4329. [Google Scholar]
- 30. Bergquist C., Bridgewater B. M., Harlan C. J., Norton J. R., Friesner R. A., Parkin G., J. Am. Chem. Soc. 2000, 122, 10581–10590. [Google Scholar]
- 31. Di Saverio A., Focante F., Camurati I., Resconi L., Beringhelli T., D'Alfonso G., Donghi D., Maggioni D., Mercandelli P., Sironi A., Inorg. Chem. 2005, 44, 5030–5041. [DOI] [PubMed] [Google Scholar]
- 32. Focante F., Camurati I., Resconi L., Guidotti S., Beringhelli T., D'Alfonso G., Donghi D., Maggioni D., Mercandelli P., Sironi A., Inorg. Chem. 2006, 45, 1683–1692. [DOI] [PubMed] [Google Scholar]
- 33. Wang X., Power P. P., Angew. Chem. Int. Ed. 2011, 50, 10965–10968; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 11157–11160. [Google Scholar]
- 34. Beringhelli T., D'Alfonso G., Donghi D., Maggioni D., Mercandelli P., Sironi A., Organometallics 2003, 22, 1588–1590. [Google Scholar]
- 35.
- 35a. Erős G., Mehdi H., Pápai I., Rokob T. A., Király P., Tárkányi G., Soós T., Angew. Chem. Int. Ed. 2010, 49, 6559–6563; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 6709–6713; [Google Scholar]
- 35b. Dorkó É., Szabó M., Kótai B., Pápai I., Domján A., Soós T., Angew. Chem. Int. Ed. 2017, 56, 9512–9516; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 9640–9644; [Google Scholar]
- 35c. Fasano V., Ingleson M. J., Chem. Eur. J. 2017, 23, 2217–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sava A., Kegyes K. T., Popuş B. T., Dan B. C., Silvestru C., Raţ C. I., CrystEngComm 2020, 22, 8285–8289. [Google Scholar]
- 37. Olaru M., Beckmann J., Raţ C. I., Organometallics 2014, 33, 3012–3020. [Google Scholar]
- 38. Parks D. J., Piers W. E., Yap G. P. A., Organometallics 1998, 17, 5492–5503. [Google Scholar]
- 39. Samigullin K., Bolte M., Lerner H.-W., Wagner M., Organometallics 2014, 33, 3564–3569. [Google Scholar]
- 40. Khalimon A. Y., Piers W. E., Blackwell J. M., Michalak D. J., Parvez M., J. Am. Chem. Soc. 2012, 134, 9601–9604. [DOI] [PubMed] [Google Scholar]
- 41.CCDC2025883 (1), 2025884 (2), 2025885 (3), 2025886 (4) and 2025887 (1⋅H2O⋅2(CH3)2CO) contain(s) the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service www.ccdc.cam.ac.uk/structures.
- 42. Beckett M. A., Strickland G. C., Holland J. R., Varma S. K., Polymer 1996, 37, 4629–4631. [Google Scholar]
- 43. Kögel J. F., Timoshkin A. Y., Schröder A., Lork E., Beckmann J., Chem. Sci. 2018, 9, 8178–8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Böhrer H., Trapp N., Himmel D., Schleep M., Krossing I., Dalton Trans. 2015, 44, 7489–7499. [DOI] [PubMed] [Google Scholar]
- 45. Erdmann P., Leitner J., Schwarz J., Greb L., ChemPhysChem 2020, 21, 987–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


