Abstract
Purpose of the Review
Skin disease is associated with obstructive sleep apnea (OSA) both epidemiologically and mechanistically. In this review we highlight conditions which have a well-established link to obstructive sleep apnea, such as psoriasis and atopic dermatitis.
Recent findings
We describe putative mechanistic links between OSA and skin disease involving inflammatory pathways, obesity, mechanical upper airways obstruction, and hypoxia. In the context of these mechanisms we describe specific skin conditions, and other conditions which are associated with both skin manifestations (including hair/nail findings) and OSA. The risks/ benefits of CPAP in the context of skin disease are also reviewed.
Summary
We conclude that further research is needed to understand the mechanisms behind the associations between OSA and skin disease. Given the frequent co-occurrence of OSA and skin conditions, there would be great benefit for OSA clinical trials to consider improvement in skin disease as an outcome measure.
Keywords: sleep apnea, psoriasis, atopic dermatitis, sleep disturbance, obesity
Introduction
Skin diseases are a common comorbidity of sleep apnea, and sleep apnea itself can deteriorate the skin. In fact, one study found that post-treatment versus pre-treatment photos of obstructive sleep apnea (OSA) patients were deemed significantly more youthful and more attractive.1 Indeed, beauty sleep is an appropriately coined term. As far as the epidemiologic association of sleep and skin, the Danish National Registry evaluated 19,438 patients with obstructive sleep apnea (OSA) compared to age/gender matched controls, and analyzed comorbid diagnoses within 3 years of the OSA diagnosis. They found there were increased odds of skin conditions in OSA patients (OR 1.18 [1.07–1.30]).2 This finding was also affirmed in a pediatric-only cohort from the same research group with an OR of 1.32, 95% CI 1.02 to 1.71.3 To dive into this association, we conducted a scoping review of the literature. The search was conducted in Pubmed MEDLINE in November, 2019 using a combination of keywords and Medical Subject Headings (MeSH), limited to English articles only. The following search strategy was used: (“Dermatology”[Mesh] OR “Skin”[Mesh] OR “Skin Diseases”[Mesh] OR skin OR dermat*) AND (“obstructive sleep apnea” OR “Sleep Apnea, Obstructive”[Mesh]) AND English[lang].
In our review of the literature in combination with our experience, we identified a few common mechanisms linking skin disease to sleep apnea.
Inflammatory pathways: Direct mechanistic link between OSA and skin disease, via inflammatory pathways, such as with OSA and psoriasis. The inflammation induced by sleep disturbance from OSA, such as increased systemic IL-1, IL-6 and IL-12, and decreased IL-10 further exacerbate psoriasis. Psoriasis also increases TNF-alpha and IL-17 levels, which are associated with increased atherosclerotic risk and possibly OSA itself.4
Obesity: Several skin conditions are associated with obesity, which provides the mechanistic link with increased OSA risk. Diabetes is a metabolic condition that can result from obesity and is associated with skin disease, such as diabetic ulcers. OSA in these patients can further impair wound healing, perpetuating the vicious cycle of diabetic ulcers.5
Mechanical upper airway obstruction: Several syndromes and genetic conditions are associated with upper airway abnormalities as well as skin conditions. These patients are at risk for OSA. Several syndromes with lipodystrophy can also cause mechanical upper airways obstruction and OSA.
Hypoxia: Skin cancers might have an association with OSA due to induction of neovascularization signals (such as hypoxia inducible factor (HIF)-1α and/or VEGF) sent systemically in OSA as a response to intermittent hypoxia.
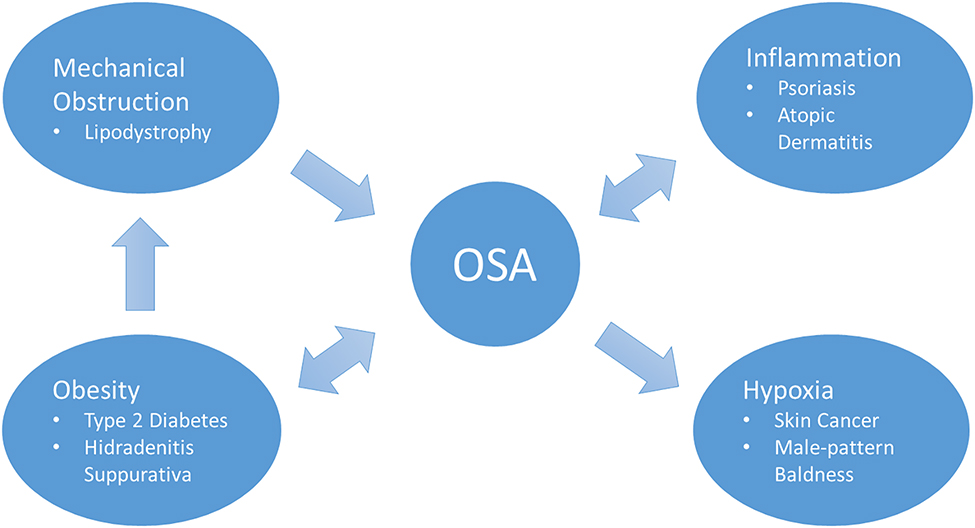
In this manuscript, we review specific skin conditions and the data behind these mechanistic associations between sleep apnea and skin. These putative relationships are outlined in Figure 1.
Figure 1:
Mechanisms linking skin disease with OSA and some example conditions
Obesity-Related Skin Conditions
Psoriasis
Psoriasis is an autoimmune skin disorder which has the most robust body of literature surrounding the epidemiologic and mechanistic link with sleep apnea. The bidirectional relationship is demonstrated epidemiologically with an increased frequency of psoriasis in OSA patients and vice versa.5 A Polish observational study found that OSA patients were four times more likely to suffer from psoriasis than the general population.6 Additionally, a Taiwanese study in 2012 that included 13,500 subjects found that OSA patients had a two-fold increase in the risk of developing psoriasis over a 3-year period, and such increased risk was tied to obesity and urban dwelling individuals.7 A European study found an increased prevalence of psoriasis in OSA patients, independent of confounders including obesity and metabolic conditions.8
The increased frequency of OSA in psoriasis patients, was shown in one small study of 33 patients with psoriasis, 54.5% had OSA,9 with a follow up study in 2019 demonstrating that disease severity of psoriasis further increases this risk.10 It also appears that comorbidities such as hypertension in psoriasis patients further increase the risk for OSA.11 Systemic inflammation as a result of psoriasis/OSA, exacerbated by hypertension, is one putative mechanism. This is accompanied by autonomic activation/increased sympathetic tone.12, 13 Autonomic activation might be the result of systemic inflammation or simply increased awakenings. Increased autonomic activity in psoriasis is even speculated to result in increased incidence of restless leg syndrome, in addition to arousals.13, 14 Sleep disruption in psoriasis tends to result in increased frequency of stage N1 sleep, potentially resulting in more pharyngeal collapse.15
Several cytokines have also been identified in the pathogenesis of both diseases. Indeed, transcriptions factors such as nuclear factor-kB and hypoxia-inducible factor-1, which are activated as the result of intermittent hypoxia and resultant oxidative stress from reperfusion injury, lead to upregulation of TNF-α and IL-6 expression.4 These same cytokines are elevated in both skin and serum of psoriasis patients.7 Additionally, the frequent awakenings in OSA decrease sleep quality and might increase itching.16 This is also demonstrated in a psoriatic mouse model in which sleep deprivation exacerbated skin lesions, with increased skin kallikrein-5 and kallikrein-7, and systemic IL-6.4
Treatment studies offer further evidence of the link between psoriasis and OSA. One case study of three patients with refractory psoriasis and OSA showed that when the patients were treated with CPAP, their psoriatic lesions decreased in severity.16 While CPAP can decrease psoriatic symptoms, treatment of psoriasis as a way to improve OSA is not clear. Considering the common inflammatory pathways in OSA and psoriasis, one study used the TNF-α inhibitor, adalimumab for 8 weeks in 20 patients, and found no improvements in OSA.17 Indeed, short-term treatment with TNF-α inhibitor is unlikely to be effective. However, long-term data on the benefit of such approach for comorbid OSA is lacking.
Atopic Dermatitis
Atopic dermatitis is certainly a sleep disturbing condition, attributable in part to the hallmark symptom of atopic dermatitis, i.e., significant nocturnal pruritus.18, 19 In fact, ~60% of children with AD have sleep disturbance,18 resulting in about 1 hour of sleep lost per night in moderate/severe disease.19 Several sleep disorders, such as periodic limb movement disorder 20 and OSA are associated with AD. The epidemiologic link between atopic dermatitis and OSA is shown in a large Taiwanese retrospective study of pediatric and adult patients (1,222 patients with OSA versus 18,330 without) adjusted models demonstrated that newly diagnosed OSA patients were 1.5 times more likely to develop atopic dermatitis. Furthermore, this finding was more pronounced in children (<18 years old) with a hazard ratio of 4.01, 95% CI 1.57–10.26.21 More recently, this same database was re-analyzed to evaluate the development of OSA in patients with AD versus those without AD. They found that being newly diagnosed with AD gave a hazard ratio of 1.86, 95% CI 1.43–2.42 of also being diagnosed with OSA in the next 1–8 years.22 Even when stratifying by pediatric age groups, all were similarly likely to develop OSA.
As this paper discusses, the pro inflammatory cytokines associated with OSA, such as IL-6, are accompanied in atopic (allergic) children by TH2 cytokine upregulation. TH2 upregulation contributes to increased susceptibility to the development of atopic dermatitis, and exacerbation of existing atopic dermatitis.
Upper airways obstruction is also more common in children with atopic dermatitis, in part due to comorbid allergic rhinitis. In terms of mechanical obstruction, although there are mixed data on the association between adenotonsillar hypertrophy in atopic children,23, 24 it is clinically believed that the upregulation of leukotrienes in allergic disease might contribute to increased adenotonsillar hypertrophy putting patients with atopic dermatitis at risk for apnea.25–28 In terms of obesity, there is an association of atopic dermatitis in both children and adults with obesity.29, 30 This is another potential mechanism linking atopic dermatitis and sleep apnea.
Research in atopic dermatitis is improving screening and assessment of sleep disturbance.31 With validated and easy to use measures, sleep disturbance and sleep-related impairment as objective and patient/parent-reported outcomes can be incorporated into atopic dermatitis clinical trials.
Obesity-Related Skin Conditions
Since the increased risk of OSA in obesity is thought to be mostly related to upper airways obstruction, it is relevant to mention skin conditions that have an increased prevalence in patients with obesity. Specifically, Type 2 diabetes is the most prevalent in obese patients, and the presence of diabetes is associated with increased risk for diabetic skin ulcers in peripheral areas with poor vascular circulation, a problem that appears to be exacerbated when OSA is concurrently present.32 OSA might impose this risk due to the endothelial dysfunction, associated with this disease in addition to small fiber neuropathy.33 Interestingly, in one case series, treatment with nasal CPAP resulted in significant improvement in wound granulation in diabetic patients.34 Perhaps failure to treat sleep apnea in diabetic patients can impair wound healing and make patients more prone to skin ulcers.34
Hidradenitis suppurativa is an obesity- associated condition due to inflammation and infection of sweat glands. OSA incidence in one cohort of patients with this disease was 3.5%, as compared to 2.5% in the control obese population. The risk was further increased in women and younger patients.35
Other common obesity-related conditions in women associated with OSA that have skin manifestation include polycystic ovarian syndrome (acne and hirsutism) and acanthosis nigricans (dark velvety patches behind neck and other folds).36, 37
Our literature review also uncovered several rare conditions which are associated with OSA and skin conditions due to the obesity link, such as Elephantiasis Nostras Verrucosa (chronic lymphedema)38 and Klinefelter's syndrome (lower extremity ulceration).39
Mechanical Upper Airway Obstruction due to associated skin conditions
Lipodystrophy, an abnormal redistribution of fat, and has also been implicated in a higher risk of OSA due to mechanical obstruction of the airway in Klinefelter's syndrome patients. The genetic subtype, familial partial lipodystrophy, causes loss of peripheral fat and accumulation in the head and neck, the predilection for these locations favoring the occurrence of OSA.40 Lipodystrophy resulting from certain HIV medications, specifically protease-inhibitors, is another causal factor.41 In these patients, central fat accumulation posterior to the dorsocervical spine may develop, and may explain why 7% of HIV patients have OSA versus 2–4% in the normal population.42 Multiple Symmetrical Lipomatosis, also known as the Launois-Bensaude Syndrome is another rare condition that is associated with OSA.43
A 142 patient case series sought to look at the upper airway and digestive manifestations of the mucosal bullous disorder called cicatricial pemphigoid. While 79% of patients were found to have nasal lesions, 24% of these caused nasal obstruction and two patients were diagnosed clinically with sleep apnea due to the upper airway obstruction.44
Hypoxia- association with skin conditions
Skin cancer and OSA
A less commonly thought of association with obstructive sleep apnea is skin cancer, supported by a relative small amount of data linking to melanoma. One US nationwide cohort study of 5.6 million people in an employee health insurance database found increased odds of melanoma in patients with OSA (OR 1.14, CI 1.10–1.18).45 A few studies have also evaluated potential mechanisms of this association. In one Spanish cohort study of 360 newly diagnosed melanoma patients, stratified by apnea-hypopnea index (AHI), full adjusted models demonstrated of biomarkers tested, VCAM-1 in both mild OSA (AHI 5–15) and moderate/severe OSA (AHI >15) was significantly increased compared to no OSA (OR 2.07, CI 1.12–3.89, p=0.021 versus 2.35, CI 1.20–4.66, p=0.013, respectively).46 In a prospective Spanish cohort study of 376 patients with cutaneous melanoma undergoing polysomnography, they found that nocturnal intermittent hypoxia as computed using a desaturation index, has a small association with tumor-derived hypoxia inducible factor (HIF)-1α (OR 1.03 (95% CI: 1.01–1.06)), but not vascular endothelial growth factor (VEGF).47 Furthermore, in 436 consecutively recruited patients the aggressiveness of cutaneous melanoma assessed through various well-defined criteria such as Breslow index was particularly and independently increased in patients with OSA, particularly if they were younger.48, 49
Other
Another OSA-associated skin/hair condition in which the mechanism is suggested to be hypoxia related is male patterned baldness. In a cross-sectional study of 932 men, men with both OSA and a family history of hair loss had a seven-fold increase in odds for having male-pattern baldness compared to those without both risk factors (CI 3.70–12.56). The authors theorized that such phenomenon may be related to the chronic-intermittent hypoxia of OSA which disrupts the normal division of hair follicles, and causes iron deposition in tissues and decreased transferrin saturation, resulting in inadequate iron availability to support dividing follicles.50
Skin-related consequences of non-invasive positive pressure ventilation, the bad and the good
As the reader is well aware, treatment of obstructive sleep apnea is often administered through various types of masks which deliver non-invasive continuous positive airway pressure during sleep. In one study, up to 50% of CPAP users reported skin allergy, air leaks or abrasions, however this generally is not severe enough to limit use of treatment.51 The masks can result in various types of dermatitis. Allergic contact dermatitis is a more severe type of dermatitis which is associated with itching, redness and, if bad enough, blistering. Silicone can in rare cases be an allergen inducing allergic contact dermatitis and has been reported in silicone-based CPAP masks to result in allergic contact dermatitis.51 Irritant dermatitis is less likely to itch and does not blister, but can result in redness and irritation simply from the physical contact of any CPAP mask on the skin. Patients with underlying dry skin are at increased risk for this problem. Treatment with moisturizers, such as Aquaphor or Vaseline prior to CPAP usage might protect the face from CPAP-mask induced irritant dermatitis. Incorrect usage of CPAP masks also put patients at higher risk for skin drying due to air leakage around the mask.
Importantly, CPAP might also be a skin therapeutic as adequately treated OSA can improve wound healing. In one case study, resolution of hand dermatitis occurred with CPAP usage. The authors speculated that the improved tissue oxygenation and reduction in sympathetic tone (due to decreased nocturnal awakenings) was the reason for hand dermatitis improvement.52 Two patients with sleep apnea were found to have yellow nail syndrome, and one patient’s nail discoloration resolved with CPAP therapy.53 Another adult was found to have onychophagia, and after treatment with positive pressure (potentially improving this parasomnia in this patient) the nail biting and resultant onychodystrophy stopped.53
Conclusion
In conclusion, skin diseases have several epidemiologic associations with OSA. There are 4 putative mechanisms we propose with some evidence to support them, to link most skin conditions with OSA. 1) Bidirectional systemic inflammation associated with OSA and inflammatory skin disease (most well described in psoriasis), in which each condition exacerbates the other. 2) Obesity itself and obesity-associated conditions (such as type-2 diabetes) in which skin manifestations and concurrent OSA are commonly observed. Type 2 diabetes is an interesting example, because OSA is thought to induce poor wound healing, and can contribute to festering diabetic ulcers. 3) Mechanical upper airway obstruction can result from obesity, as well as other specific conditions, such as lipodystrophy in which fat deposits can occur in the airway. 4) Hypoxia related to OSA appears to be associated with skin cancers, possibly related to neovascular proliferative signals related to the intermittent hypoxia. Underlying these mechanisms is also autonomic dysfunction as a consequence of OSA, whereby the presence of increased sympathetic tone from frequent nighttime awakenings and recurrent hypoxia are important contributors. Excessive sympathetic outflow to skin may accelerate aging and unmask or exacerbate pre-existing skin conditions. Continuous Positive Airway Pressure can be associated with skin irritation, irritant dermatitis and rarely allergic contact dermatitis. However, the benefits of CPAP most definitely outweigh the risks. Further research is needed to better describe the mechanisms underlying the clinical associations between skin diseases and obstructive sleep apnea, and the potential therapeutic effects on skin disease by the treatment of OSA either by CPAP or other interventions.
Acknowledgements
The authors would like to acknowledge Ms. Andrea Fawcett for her help in the literature review.
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest: Dr. Fishbein has grant support from Pfizer and served as consultant for Regeneron.
Human and Animal Rights
This article does not contain any studies with human or animal subjects performed by any of the authors.
References
- 1.Chervin RD, Ruzicka DL, Vahabzadeh A, Burns MC, Burns JW, Buchman SR. The face of sleepiness: improvement in appearance after treatment of sleep apnea. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2013;9(9):845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jennum P, Ibsen R, Kjellberg J. Morbidity prior to a diagnosis of sleep-disordered breathing: a controlled national study. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2013;9(2):103–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jennum P, Ibsen R, Kjellberg J. Morbidity and mortality in children with obstructive sleep apnoea: a controlled national study. Thorax. 2013;68(10):949–54. [DOI] [PubMed] [Google Scholar]
- 4.Hirotsu C, Nogueira H, Albuquerque RG, Tomimori J, Tufik S, Andersen ML.The bidirectional interactions between psoriasis and obstructive sleep apnea. International journal of dermatology. 2015;54(12):1352–8. [DOI] [PubMed] [Google Scholar]
- 5.Gupta MA, Simpson FC, Vujcic B, Gupta AK. Obstructive sleep apnea and dermatologic disorders. Clin Dermatol. 2017;35(3):319–27. [DOI] [PubMed] [Google Scholar]
- 6.Gabryelska A, Sochal M, Wasik B, Bialasiewicz P. Patients With Obstructive Sleep Apnea Are Over Four Times More Likely to Suffer From Psoriasis Than the General Population. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2018;14(1):153.* Several epidemiologic studies have demonstrated the association between OSA and psoriasis. This is the most recent study to add to that literature, this letter to the editor found psoriasis in 8.7% of 245 OSA patients (AHI >5) undergoing PSG.
- 7.Yang YW, Kang JH, Lin HC. Increased risk of psoriasis following obstructive sleep apnea:a longitudinal population-based study. Sleep Med 2012;13(3):285–9. [DOI] [PubMed] [Google Scholar]
- 8.Papadavid E, Dalamaga M, Vlami K, Koumaki D, Gyftopoulos S, Christodoulatos GS, et al. Psoriasis is associated with risk of obstructive sleep apnea independently from metabolic parameters and other comorbidities: a large hospital-based case-control study. Sleep Breath. 2017;21(4):949–58. [DOI] [PubMed] [Google Scholar]
- 9.Karaca S, Fidan F, Erkan F, Nural S, Pinarci T, Gunay E, et al. Might psoriasisbea risk factor for obstructive sleep apnea syndrome? Sleep Breath. 2013;17(1):275–80. [DOI] [PubMed] [Google Scholar]
- 10.Kabeloglu Ilbay V, Tas B, Altuntas M, Atakli HD, Soysal A. Risk of Obstructive Sleep Apnea Syndrome in Psoriasis Patients. Arch Iran Med 2019;22(3):137–43. [PubMed] [Google Scholar]
- 11.Papadavid E, Vlami K, Dalamaga M, Giatrakou S, Theodoropoulos K, Gyftopoulos S, et al. Sleep apnea as a comorbidity in obese psoriasis patients: a cross-sectional study. Do psoriasis characteristics and metabolic parameters play a role? J Eur Acad Dermatol Venereol. 2013;27(7):820–6. [DOI] [PubMed] [Google Scholar]
- 12.Fishbein AB, Sheldon S, Grimaldi D. Author response. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2020;16(3):465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta MA, Simpson FC, Gupta AK. Psoriasis and sleep disorders: A systematic review. Sleep Med Rev. 2016;29:63–75. [DOI] [PubMed] [Google Scholar]
- 14.Saçmaci H, Gürel G. Sleep disorders in patients with psoriasis: across-sectional study using non-polysomnographical methods. Sleep Breath. 2019;23(3):893–8. [DOI] [PubMed] [Google Scholar]
- 15.Gabryelska A, Białasiewicz P. Is Autonomic Activation a Middleman Between Obstructive Sleep Apnea Syndrome and Psoriasis? Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2018;14(6):1087–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gowda S, Goldblum OM, McCall WV, Feldman SR. Factors affecting sleep quality in patients with psoriasis. Journal of the American Academy of Dermatology. 2010;63(1):114–23. [DOI] [PubMed] [Google Scholar]
- 17.Maari C, Bolduc C, Nigen S, Marchessault P, Bissonnette R. Effect of adalimumab on sleep parameters in patients with psoriasis and obstructive sleep apnea: a randomized controlled trial. J Dermatolog Treat. 2014;25(1):57–60. [DOI] [PubMed] [Google Scholar]
- 18.Fishbein AB, Vitaterna O, Haugh IM, Bavishi AA, Zee PC, Turek FW, et al. Nocturnal eczema: Review of sleep and circadian rhythms in children with atopic dermatitis and future research directions. The Journal of allergy and clinical immunology. 2015;136(5):1170–7. [DOI] [PubMed] [Google Scholar]
- 19.Fishbein AB, Mueller K, Kruse L, Boor P, Sheldon S, Zee P, et al. Sleep disturbance in children with moderate/severe atopic dermatitis: A case-control study. Journal of the American Academy of Dermatology. 2018;78(2):336–41. [DOI] [PubMed] [Google Scholar]
- 20.Treister AD, Stefek H, Grimaldi D, Rupani N, Zee P, Yob J, et al. Sleep and Limb Movement Characteristics of Children With Atopic Dermatitis Coincidentally Undergoing Clinical Polysomnography. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2019;15(8):1107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tien KJ, Chou CW, Lee SY, Yeh NC, Yang CY, Yen FC, et al. Obstructive sleep apnea and the risk of atopic dermatitis: a population-based case control study. PLoS One. 2014;9(2):e89656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu JM, Lin CS, Chen SJ, Chen CY, Lin CL, Kao CH. Association between obstructive sleep apnea and atopic dermatitis in children: A nationwide, population-based cohort study. Pediatr Allergy Immunol. 2018;29(3):260–6.* This is the most recent epidemiologic publication linking atopic dermatitis with OSA, and to our knowledge the largest pediatric cohort study including 120,736 AD patients and 120,736 age/gender matched controls. They found atopic dermatitis patients have a 1.86 fold risk of OSA.
- 23.Alexopoulos EI, Bizakis J, Gourgoulianis K, Kaditis AG. Atopy does not affect the frequency of adenotonsillar hypertrophy and sleep apnoea in children who snore. Acta paediatrica (Oslo, Norway: 1992). 2014;103(12):1239–43. [DOI] [PubMed] [Google Scholar]
- 24.Huo Z, Shi J, Shu Y, Xiang M, Lu J, Wu H. The relationship between allergic status and adenotonsillar regrowth: a retrospective research on children after adenotonsillectomy. Scientific reports. 2017;7:46615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nagaya T, Kawata K, Kamekura R, Jitsukawa S, Kubo T, Kamei M, et al. Lipid mediators foster the differentiation of T follicular helper cells. Immunol Lett. 2017;181:51–7. [DOI] [PubMed] [Google Scholar]
- 26.Paulucci BP, Pereira J, Picciarelli P, Levy D, Di Francesco RC. Expression of cysteinyl leukotriene receptor 1 and 2 (CysLTR1 and CysLTR2) in the lymphocytes of hyperplastic tonsils: comparison between allergic and nonallergic snoring children. Int Forum Allergy Rhinol. 2016;6(11):1151–8. [DOI] [PubMed] [Google Scholar]
- 27.Kheirandish-Gozal L, Bandla HP, Gozal D. Montelukast for Children with Obstructive Sleep Apnea: Results of a Double-Blind, Randomized, Placebo-Controlled Trial. Ann Am Thorac Soc. 2016;13(10):1736–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dayyat E, Serpero LD, Kheirandish-Gozal L, Goldman JL, Snow A, Bhattacharjee R, et al. Leukotriene pathways and in vitro adenotonsillar cell proliferation in children with obstructive sleep apnea. Chest. 2009;135(5):1142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Silverbergrg JI, Becker L, Kwasny M, Menter A, Cordoro KM, Paller AS. Central obesity and high blood pressure in pediatric patients with atopic dermatitis. JAMA dermatology. 2015;151(2):144–52. [DOI] [PubMed] [Google Scholar]
- 30.Silverberg JI, Silverberg NB, Lee-Wong M. Association between atopic dermatitis and obesity in adulthood. The British journal of dermatology. 2012;166(3):498–504. [DOI] [PubMed] [Google Scholar]
- 31.Fishbein AB, Lor J, Penedo FJ, Forrest CB, Griffith JW, Paller AS. Patient-Reported Outcomes for Measuring Sleep Disturbance in Pediatric Atopic Dermatitis: cross sectional study of PROMIS Pediatric Sleep Measures and Actigraphy. Journal of the American Academy of Dermatology. 2020.** This is the first study to present an evidence-based algorithm for assessing sleep in children with atopic dermatitis, first by screening with patient/parent-reported measures and then objective sleep assessment as needed.
- 32.Tahrani AA, Ali A, Raymond NT, Begum S, Dubb K, Mughal S, et al. Obstructive sleep apnea and diabetic neuropathy: a novel association in patients with type 2 diabetes. American journal of respiratory and critical care medicine. 2012;186(5):434–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Altaf QA, Ali A, Piya MK, Raymond NT, Tahrani AA. The relationship between obstructive sleep apnea and intra-epidermal nerve fiber density, PARP activation and foot ulceration in patients with type 2 diabetes. Journal of diabetes and its complications. 2016;30(7):1315–20. [DOI] [PubMed] [Google Scholar]
- 34.Vas PR, Ahluwalia R, Manas AB, Manu CA, Kavarthapu V, Edmonds ME. Undiagnosed severe sleep apnoea and diabetic foot ulceration - a case series based hypothesis: a hitherto under emphasized factor in failure to heal. Diabetic medicine: a journal of the British Diabetic Association. 2016;33(2):e1–4. [DOI] [PubMed] [Google Scholar]
- 35.Wertenteil S, Strunk A, Garg A. Incidence of obstructive sleep apnoea in patients with hidradenitis suppurativa: a retrospective population-based cohort analysis. The British journal of dermatology. 2018;179(6):1398–9. [DOI] [PubMed] [Google Scholar]
- 36.Setji TL, Brown AJ. Polycystic ovary syndrome: diagnosis and treatment. Am J Med. 2007;120(2):128–32. [DOI] [PubMed] [Google Scholar]
- 37.Hoppin AG, Katz ES, Kaplan LM, Lauwers GY. Case records of the Massachusetts General Hospital. Case 31–2006. A 15-year-old girl with severe obesity. N Engl J Med. 2006;355(15):1593–602. [DOI] [PubMed] [Google Scholar]
- 38.Buyuktas D, Arslan E, Celik O, Tasan E, Demirkesen C, Gundogdu S. Elephantiasis nostras verrucosa on the abdomen of a Turkish female patient caused by morbid obesity. Dermatology online journal. 2010;16(8):14. [PubMed] [Google Scholar]
- 39.Shanmugam VK, Tsagaris KC, Attinger CE. Leg ulcers associated with Klinefelter's syndrome: a case report and review of the literature. International wound journal. 2012;9(1):104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hegele RA, Al-Attar SA, Rutt BK. Obstructive sleep apnea in 2 women with familial partial lipodystrophy due to a heterozygous LMNA R482Q mutation. Cmaj. 2007;177(7):743–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carr A, Cooper DA. Adverse effects of antiretroviral therapy. Lancet. 2000;356(9239):1423–30. [DOI] [PubMed] [Google Scholar]
- 42.Epstein LJ, Strollo PJ Jr., Donegan RB, Delmar J, Hendrix C, Westbrook PR. Obstructive sleep apnea in patients with human immunodeficiency virus (HIV) disease. Sleep. 1995;18(5):368–76. [DOI] [PubMed] [Google Scholar]
- 43.Harsch IA, Bergmann T, Koebnick C, Wiedmann R, Ruderich F, Hahn EG, et al. Adiponectin, resistin and subclinical inflammation--the metabolic burden in Launois Bensaude Syndrome, a rare form of obesity. Journal of physiology and pharmacology: an official journal of the Polish Physiological Society. 2007;58 Suppl 1:65–76. [PubMed] [Google Scholar]
- 44.Hanson RD, Olsen KD, Rogers RS 3rd. Upper aerodigestive tract manifestations of cicatricial pemphigoid. Ann Otol Rhinol Laryngol. 1988;97(5 Pt 1):493–9. [DOI] [PubMed] [Google Scholar]
- 45.Gozal D, Ham SA, Mokhlesi B. Sleep Apnea and Cancer: Analysis of a Nationwide Population Sample. Sleep. 2016;39(8):1493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santamaria-Martos F, Benítez I, Girón C, Barbé F, Martínez-García MA, Hernández L, et al. Biomarkers of carcinogenesis and tumour growth in patients with cutaneous melanoma and obstructive sleep apnoea. Eur Respir J. 2018;51(3). [DOI] [PubMed] [Google Scholar]
- 47.Almendros I, Marítnez-García M, Campos-Rodríguez F, Riveiro-Falkenbach E, Rodríguez-Peralto JL, Nagore E, et al. Intermittent Hypoxia Is Associated With High Hypoxia Inducible Factor-1α but Not High Vascular Endothelial Growth Factor Cell Expression in Tumors of Cutaneous Melanoma Patients. Front Neurol. 2018;9:272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez-Garcia MA, Campos-Rodriguez F, Nagore E, Martorell A, Rodriguez-Peralto JL, Riveiro-Falkenbach E, et al. Sleep-Disordered Breathing Is Independently Associated With Increased Aggressiveness of Cutaneous Melanoma: A Multicenter Observational Study in 443 Patients. Chest. 2018;154(6):1348–58.** First study to show SDB is associated with melanoma aggressiveness. This well-designed study of 443 melanoma patients evaluated objective sleep disordered breathing measures (such as AHI) and patient-reported measures to demonstrate the association of SDB and several markers of melanoma aggressiveness (i.e. ulceration, sentinel lymph noted spread, etc). There was a “severity dependent” effect of AHI on tumor staging.
- 49.Martinez-Garcia MA, Campos-Rodriguez F, Almendros I, Garcia-Rio F, Sanchez-de-la-Torre M, Farre R, et al. Cancer and Sleep Apnea: Cutaneous Melanoma as a Case Study. American journal of respiratory and critical care medicine. 2019;200(11):1345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baik I, Lee S, Thomas RJ, Shin C. Obstructive sleep apnea, low transferrin saturation levels, and male-pattern baldness. International journal of dermatology. 2019;58(1):67–74. [DOI] [PubMed] [Google Scholar]
- 51.Pépin JL, Leger P, Veale D, Langevin B, Robert D, Lévy P. Side effects of nasal continuous positive airway pressure in sleep apnea syndrome. Study of 193 patients in two French sleep centers. Chest. 1995;107(2):375–81. [DOI] [PubMed] [Google Scholar]
- 52.Matin A, Bliwise DL, Wellman JJ, Ewing HA, Rasmuson P. Resolution of dyshidrotic dermatitis of the hand after treatment with continuous positive airway pressure for obstructive sleep apnea. South Med J. 2002;95(2):253–4. [PubMed] [Google Scholar]
- 53.Nino G, Singareddy R. Severe onychophagia and finger mutilation associated with obstructive sleep apnea. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2013;9(4):379–81. [DOI] [PMC free article] [PubMed] [Google Scholar]