Figure 3. Pre-existing SARS-CoV-2 neutralizing antibody responses are boosted by a single dose of a spike-derived mRNA vaccine.
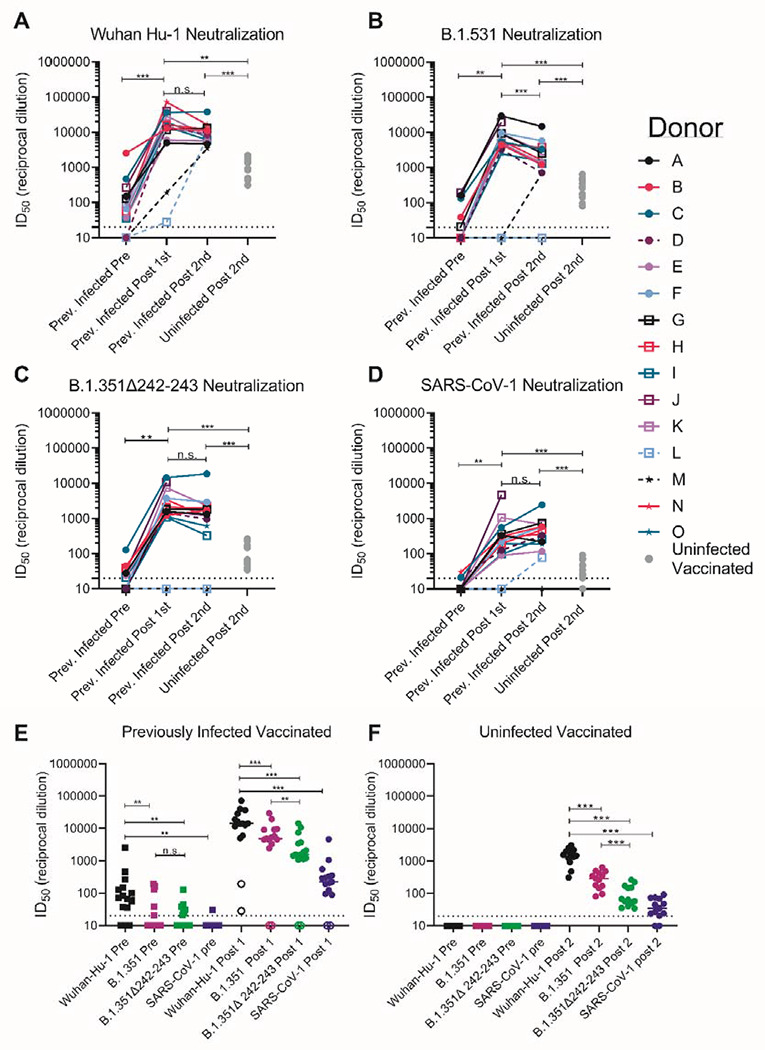
The serum dilution resulting in 50% neutralization (ID50) of (A) Wuhan-Hu-1, (B) B.1.351, (C) B.1.351Δ242-243, and (D) SARS-CoV-1 pseudoviruses was measured in recovered COVID-19 donors prior to and following a one or two immunizations with the Pfizer/BioNTech or Moderna vaccines, and in uninfected donors following two vaccine doses as indicated. Data points between previously infected donors who were symptomatic and asymptomatic are connected by solid and dashed lines, respectively in A-D. (E) Serum dilution resulting in 50% neutralization (ID50) from recovered donors prior to (squares) and following a single immunization (circles) with the Pfizer/BioNTech or Moderna vaccines against Wuhan-Hu-1, B.1.351, B.1.351Δ242-243 and SARS-CoV-1 pseudoviruses as indicated. Previoulsy infected donors who were asymptomatic, negative for anti-IgG RBD antibodies, and RBD-specific IgG+ memory B cells prior to vaccination are shown as open circles. (F) Neutralizing potency (ID50) of serum from uninfected donors following two immunizations with the Pfizer/BioNTech or Moderna vaccines against the indicated pseudoviruses. Each data point represents a different donor and the horizonal bars represent the medians in E and F. The dashed lines demarcate the lowest serum dilutions tested. Experiments were performed once. Significant differences in infected donors before or after vaccination, or from the same timepoint against different variants (*p<0.05, ** p<0.01 and ***p<0.001) were determined using a Wilcoxon signed rank test. Significant differences between previously infected and uninfected donors (*p<0.05, **p<0.01 and ***p<0.001) were determiend using a Wilcoxon rank sum test.
