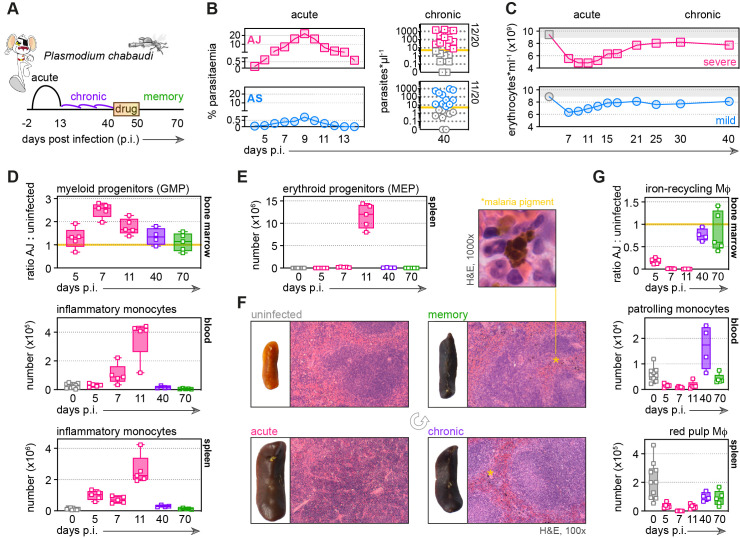
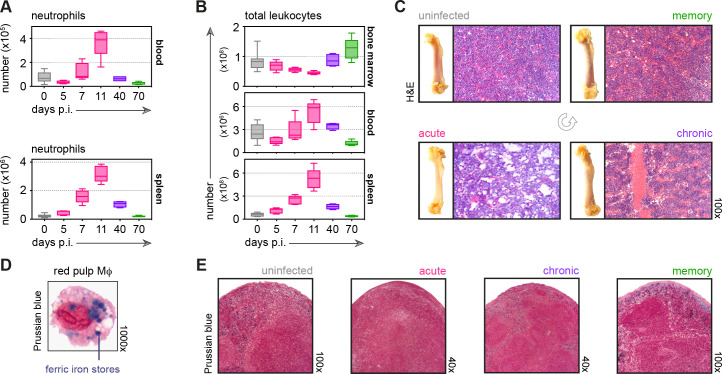
Figure 1. Malaria triggers emergency myelopoiesis and obliterates tissue-resident macrophages.
(A) C57Bl/6 mice were infected with Plasmodium chabaudi AJ or AS sporozoites; the blood-stage of infection started 2 days later after the release of merozoites from the liver. Mice were chronically infected for 40 days, at which point we administered the antimalarial drug chloroquine. Memory responses were assessed 30 days thereafter. Note that we exclusively use days post infection (p.i.) to refer to the blood-stage of malaria. (B) Acute parasitaemia was monitored daily using Giemsa stained thin blood films and chronic infection was verified 40 days p.i. by qPCR (n = 20 per group). Symbols below the limit of detection (5 parasites*μl−1) are coloured grey and these mice were excluded from the study. (C) The mean number of erythrocytes*ml−1 is shown before (grey symbols) and during infection (n = 10 for AJ and n = 14 for AS). Severe anaemia is defined as >50% loss of red cells. (D and E) Inflammatory monocytes and progenitor cells (granulocyte monocyte progenitors [GMP] and megakaryocyte erythroid progenitors [MEP]) from uninfected mice (0 days p.i.), AJ-infected mice (5, 7, 11, and 40 days p.i.) and once-infected mice (memory, 70 days p.i.) were analysed by flow cytometry (n = 4–5 per time point, box-plots show median and IQR). Uninfected age-matched controls were analysed at each time point and pooled for graphing (n = 10); absolute counts are shown for blood and spleen. In (D), GMP are shown as a ratio of infected:uninfected at each time point because bone marrow cellularity increases with age. (F) Paraffin-embedded spleen sections were H&E stained (11 days p.i. for acute AJ infection) – examples of malaria pigment in chronically infected and once-infected mice are marked with an asterisk. (G) Tissue-resident macrophages (Mɸ) and patrolling monocytes from uninfected mice, AJ-infected mice, and once-infected mice were analysed by flow cytometry (n = 4–5 per time point, box-plots show median and IQR). Absolute counts (blood and spleen) and cell ratios (bone marrow) are shown exactly as described for (D and E). See Supplementary file 1 for all antibody panels and gating strategies.