Figure 1.
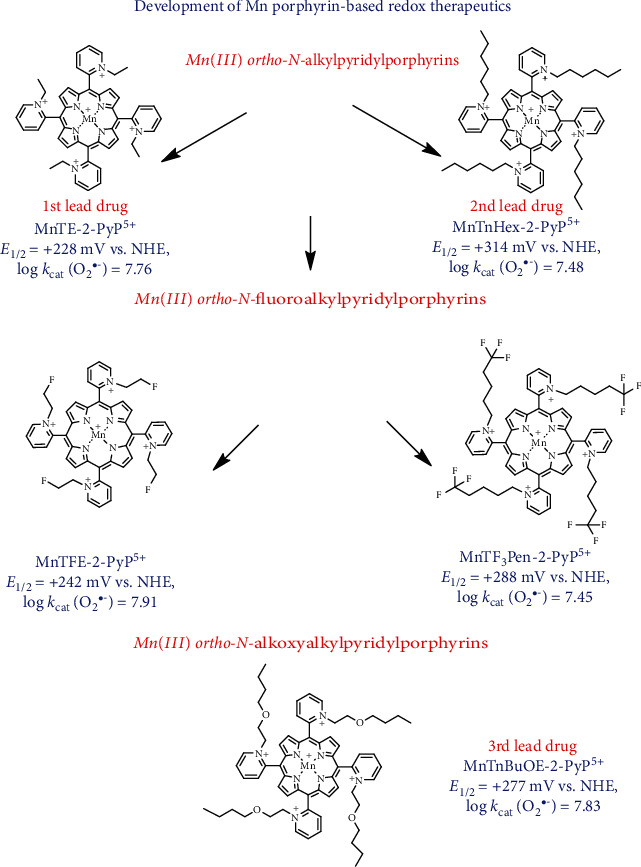
Development of Mn porphyrins towards analogs of optimized efficacy, bioavailability, and safety/toxicity [4, 25]. Ortho positions of N-pyridyl substituents, due to the vicinity to the Mn site and the cationic charge, afford the best thermodynamics and kinetics for the catalysis of O2•- dismutation in an SOD-like fashion; we named the key feature of such design the ortho effect. Based on the ortho effect, the first generation of powerful SOD mimics was synthesized [6, 26]. Among those, a hydrophilic, MnTE-2-PyP5+ (BMX-010), became our 1st lead drug. Its ethylpyridyl chains were subsequently lengthened from up to 8 carbon atoms in order to increase the lipophilicity of the molecule [7]. Among those compounds, MnTnHex-2-PyP5+ has the best balance between the lipophilicity (that would not damage cellular membranes at low clinically relevant concentrations) and its SOD-like potency and became our 2nd lead drug. To further diminish the toxicity of MnTnHex-2-PyP5+, we introduced the oxygen atoms into hexyl chains deep into the porphyrin cavity. The butoxyethyl analog was created, MnTnBuOE-2-PyP5+ (BMX-001), and this became our 3rd lead drug. The approach was similar to the one where ether of sodium dodecyl sulfate (SDS) was synthesized to reduce the toxicity of SDS [10]. Based on the same principles of the ortho effect, the N-alkylpyridyl groups were fluorinated and two analogs were created: Mn(III) meso-tetrakis(N-fluoroethylpyridinium-2-yl)porphyrin, MnTFE-2-PyP5+ (BMX-002), and Mn(III) meso-tetrakis(N-trifluoropentylpyridinium-2-yl)porphyrin, MnTF3Pen-2-PyP5+ (BMX-003). Aqueous chemistry data suggest improved safety/toxicity and efficacy profiles of fluorinated vs. nonfluorinated analogs. Initial studies confirmed their potency in radioprotecting normal tissue and radio- and chemosensitizing the 4T1 mouse breast cancer cell line in cellular and animal models [23, 24]. Below each of the structures, the metal-centered reduction potential for the Mn(III)/Mn(II) redox couple and log kcat(O2•−) for the catalysis of O2•- dismutation are listed.
