Figure 2.
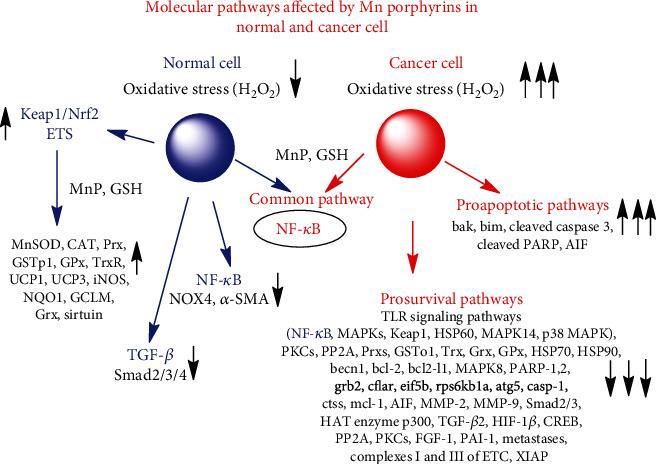
Molecular pathways affected by Mn porphyrins in normal and cancer cell. Most of the data were derived from breast cancer and glioma studies. Two major pathways, affected by Mn porphyrins, have been explored: nuclear factor-κB (NF-κB) and Keap1/nuclear factor E2-related factor 2 (Nrf2). In both tumor and normal tissue injury, NF-κB was inactivated by the MnP-driven catalysis of p50 and p65 cysteine oxidation in the presence of H2O2 and GSH. The yield of inactivation depends upon MnP and H2O2 levels, both of which are higher in tumor than in normal cell Normal cell. Hematopoietic stem/progenitor cells. The nuclear factor E2-related factor 2 (Nrf2) pathway was activated, and it upregulated endogenous antioxidative defenses such as MnSOD, catalase (CAT), glutathione peroxidase (GPx), peroxiredoxin (Prx), glutaredoxin (Grx), glutathione-S-transferase (GSTp1), NAD(P)H quinone dehydrogenase (NQO1), uncoupling proteins 1 and 3 (UCP1 and UCP3), and glutamate cysteine ligase regulatory subunit (GCLM). In the study, the ETS transcription activity, which plays role in hematopoiesis, was also increased. The ETS family of transcription factors is among the end effector molecules of the signal transduction pathway. Phosphorylation of ETS modulates DNA binding, protein-protein interaction, transcriptional activation, and subcellular localization. ETS stands for E26 transformation specific or E26, as the ETS sequence (v-ets) was first identified in the genome of the avian retrovirus E26. Normal colorectal and prostate fibroblasts. The effect of MnP on NADPH oxidase 4 (NOX4) and α-smooth muscle actin (α-SMA), Nrf2, NF-κB, and TGF-β molecular with a subsequent effect on Smad 2/3 was reported. Smad 2/3 is the main signal transducer for receptors of the transforming growth factor beta (TGF-β) superfamily. The abbreviation stands for an acronym from the fusion of Caenorhabditis elegans Sma and the Drosophila Mad (Mothers against decapentaplegic) genes. Cancer. 4T1 breast cancer. NF-κB, a part of the TLR molecular pathway, is the major pathway affected by MnP/ascorbate in 4T1 cells as found by redox proteomics. In addition, and as a part of the TLR pathway, MnP/ascorbate oxidized cysteines of different mitogen-activated protein kinases (MAPKs) (p38 mitogen-activated protein kinase (p38 MAPK) and MAPK14) and protein kinase C (PKC ι and δ). We have also seen the oxidation of Kelch-like ECH-associated protein 1 (Keap1) as part of the TLR pathway. In addition, endogenous antioxidative defenses were oxidized, such as Cu,ZnSOD, glutathione-S-transferase (GSTo1), peroxiredoxins (Prxs), thioredoxin, thioredoxin 1 (Trx1), and heat shock proteins (HSPs). In another 4T1 breast cancer study, the inactivation of c-Jun N-terminal kinase (JNK), extracellular signal-regulated kinase (ERK), and protein kinase B (AKT) by MnP/RT was seen. Also, the suppression of antiapoptotic NF-κB, bcl-2, and mcl-1; the promotion of proapoptotic pathways, bak and bim; and the increase of cleaved PARP and cleaved caspase 3 were demonstrated. Inflammatory breast cancer. In SUM149 cells, the inhibitory effect of MnTE-2-PyP5+ on NF-κB, p38 MAPK, ERK1/2, XIAP (X-linked inhibitor of apoptosis protein); the activation of apoptosis-inducing factor (AIF); and the increase in PARP cleavage were seen. Glioblastoma multiforme/glioma. In glioma tissue from a subcutaneous xenograft mouse study, the cell death array showed a number of genes downregulated by MnP/RT (fold difference vs. RT alone listed in parenthesis). Among the most affected genes are those that regulate the following proteins (listed from most affected to less affected): eif5b, eukaryotic translation initiation factor 5B (-408.8-fold); rps6kb1a, serine/threonine kinase that acts downstream of PIP3 (phosphatidylinositol (3,4,5)-trisphosphate) and phosphoinositide-dependent kinase-1 in the PI3 kinase pathway (-176.6-fold); bcl2-l1, a potent inhibitor of apoptosis (-59-fold); ctss, cathepsin S (-59-fold); grb2, growth receptor bound protein-2, an adaptor protein involved in signal transduction/cell communication (-45.5-fold); atg5, autophagy-related protein (-43-fold); cflar, CFLAR, CASP8, and FADD-like apoptosis regulator (-38.7-fold); casp1, inflammasome mediates activation of caspase-1 which promotes the secretion of the proinflammatory cytokines IL-1β, IL-18, and pyroptosis (-37-fold); CD40, cluster of differentiation 40, member of the TNF superfamily essential to a broad array of immune and inflammatory responses (-21-fold); becn1, plays critical role in autophagy and cell death (-16-fold); NF-κB p105 subunit (-13.6-fold); MAPK8 (also known as JNK1), mitogen-activated kinase which plays a key role in T cell proliferation, apoptosis, and differentiation (-7.8-fold); PARP-1 and PARP-2, poly(ADP-ribose) polymerase-1 and 2 (-5.4-fold and -3.3-fold, respectively). Prostate cancer. The effect of MnP was seen on histone acetyltransferase p300 (HAT p300), cAMP response element-binding protein (CREB), fibroblast growth factor (FGF), hypoxia-inducible factor 1β(HIF-1β), and plasminogen activator inhibitor-1 (PAI-1). Colorectal cancer. MnP-driven suppression of transforming growth factor (TGF-β) and in turn matrix-metalloproteinases 2 and 9 (MMP-2 and MMP-9) and Smad 2/3 was reported. Hematologic malignancies. NF-κB and complexes I, III, and IV of the mitochondrial electron-transport chain (ETC) were oxidized by MnP.
