Figure 2. Cardiac progenitors are expanded posteriorly within the nkx2.5+ progenitor field.
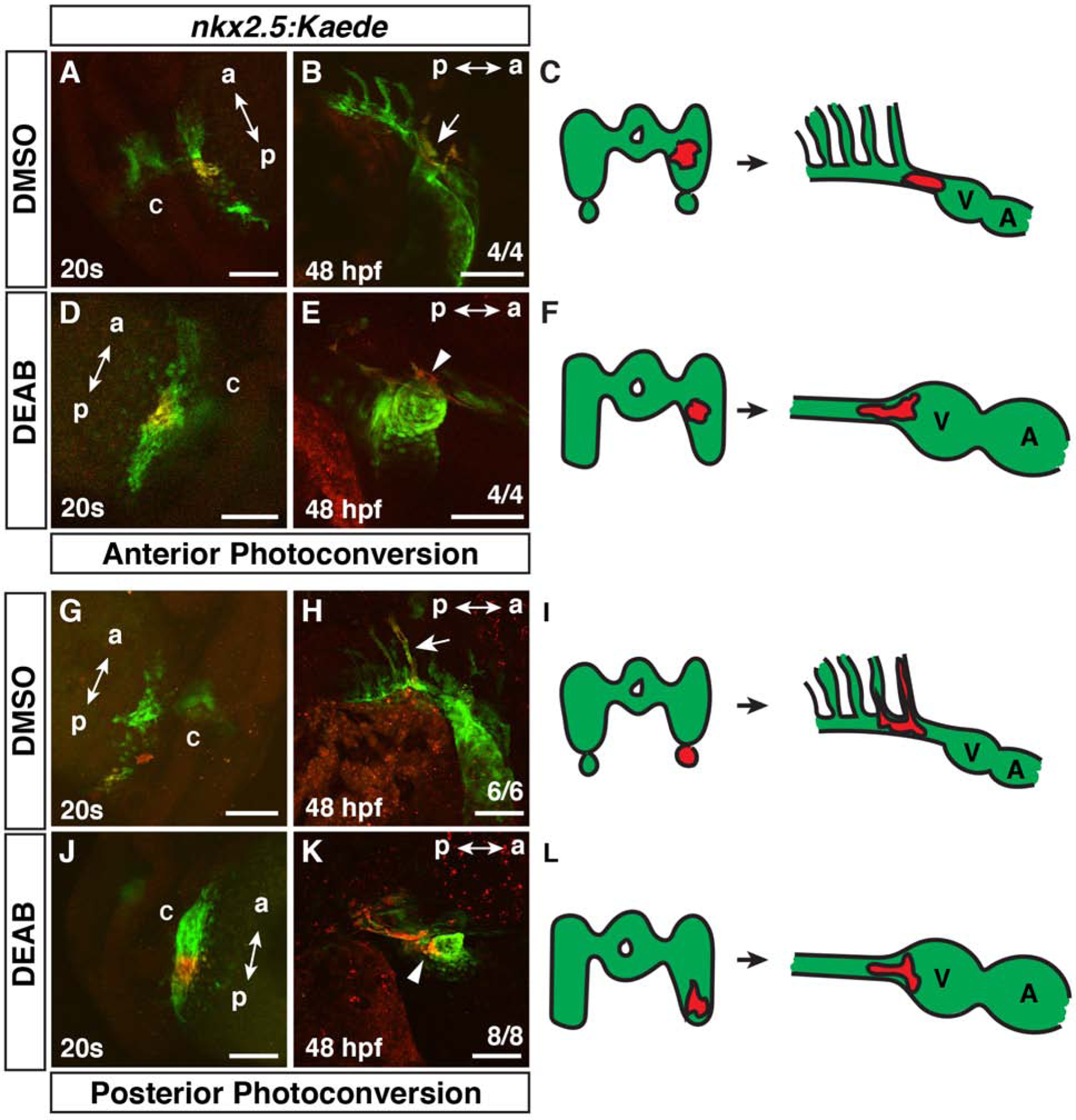
(A,B,D,E,G,H,J,K) Photoconversion-mediated lineage tracing of nkx2.5:Kaede+ progenitor cells in DMSO- and DEAB-treated embryos at the 20s stage (19 hpf) and at 48 hpf. Views in A,D,G, J are dorso-lateral with anterior up. Views in B,E,H,K are lateral with anterior right and dorsal up. a – anterior, p – posterior, c - cardiac cone. Embryos at 48 hpf (B,E,H,K) are the same embryos shown with photoconverted cells at the 20s stage (19 hpf) in (A,D,G,J). Arrow in B indicates photoconverted cells in the ventral aorta and OFT. (C,F,I,L) Schematics summarizing the lineage tracing in the anterior and posterior lateral nkx2.5:Kaede+ fields. V – ventricle, A – Atrium. In the anterior lateral nkx2.5+ region, 4 of 4 control embryos had labeled cells give rise to the ventral aorta/OFT, while 1 of the 4 also labeled the 3rd PAA. 4 of 4 DEAB-treated embryos with nkx2.5:Kaede+ cell clusters labeled in similar regions gave rise to the ventral aorta, OFT, and CMs. In the posterior lateral nkx2.5:Kaede+ field, 6 of 6 control embryos had photoconverted cells contribute to the 3rd PAA. 8 of 8 DEAB-treated embryos had photoconverted cells give rise to the ventral aorta, OFT, and CMs. Arrow in H indicates photoconverted cells in the 3rd PAA. Arrowheads in E and K indicated photoconverted CMs at the arterial pole. Scale bars – 100 μm.
