Figure 5. Inhibition of mitochondria AKT uncoupled mitochondria respiration and decreased cellular ATP in renal tubular epithelial cells.
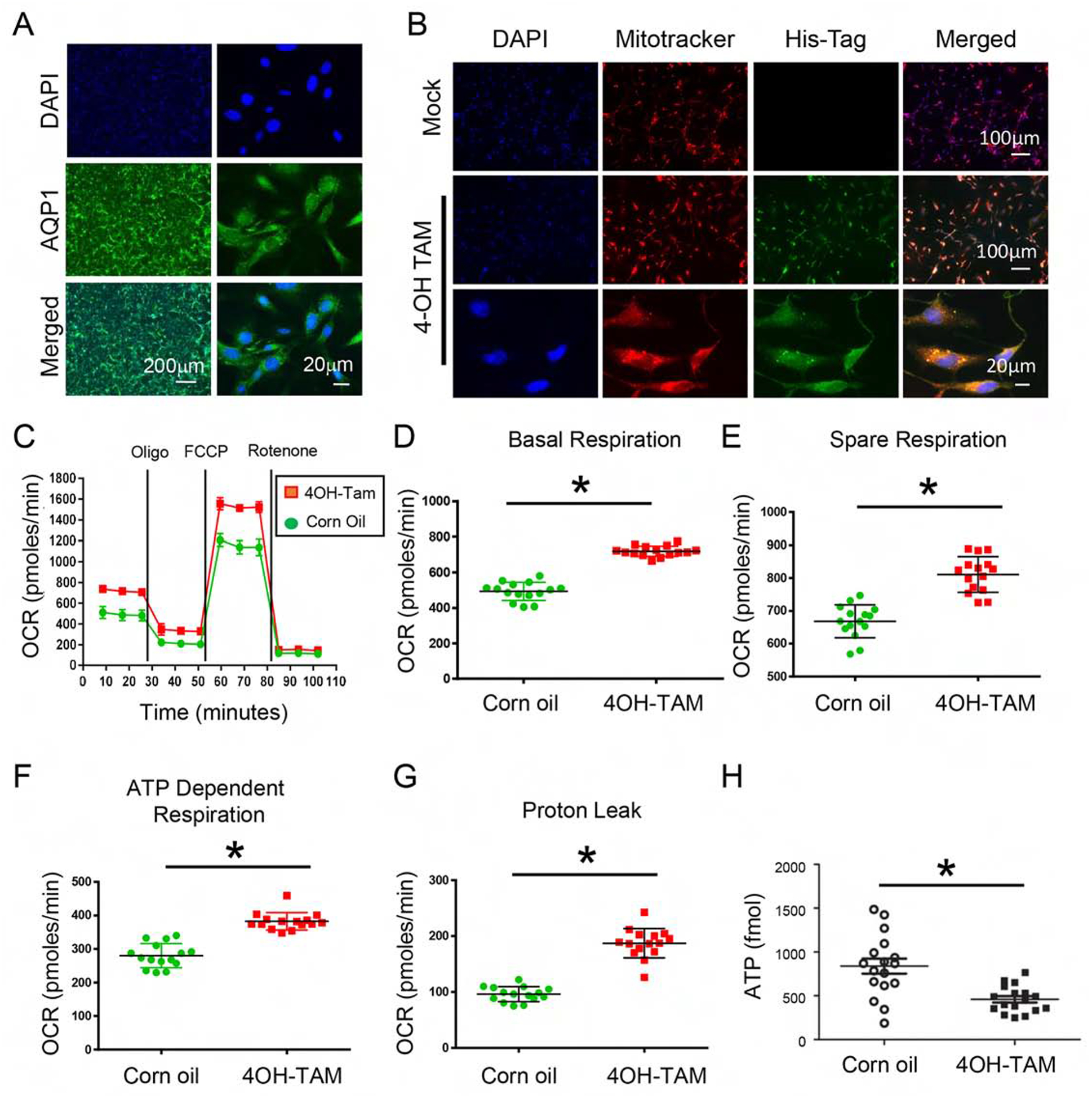
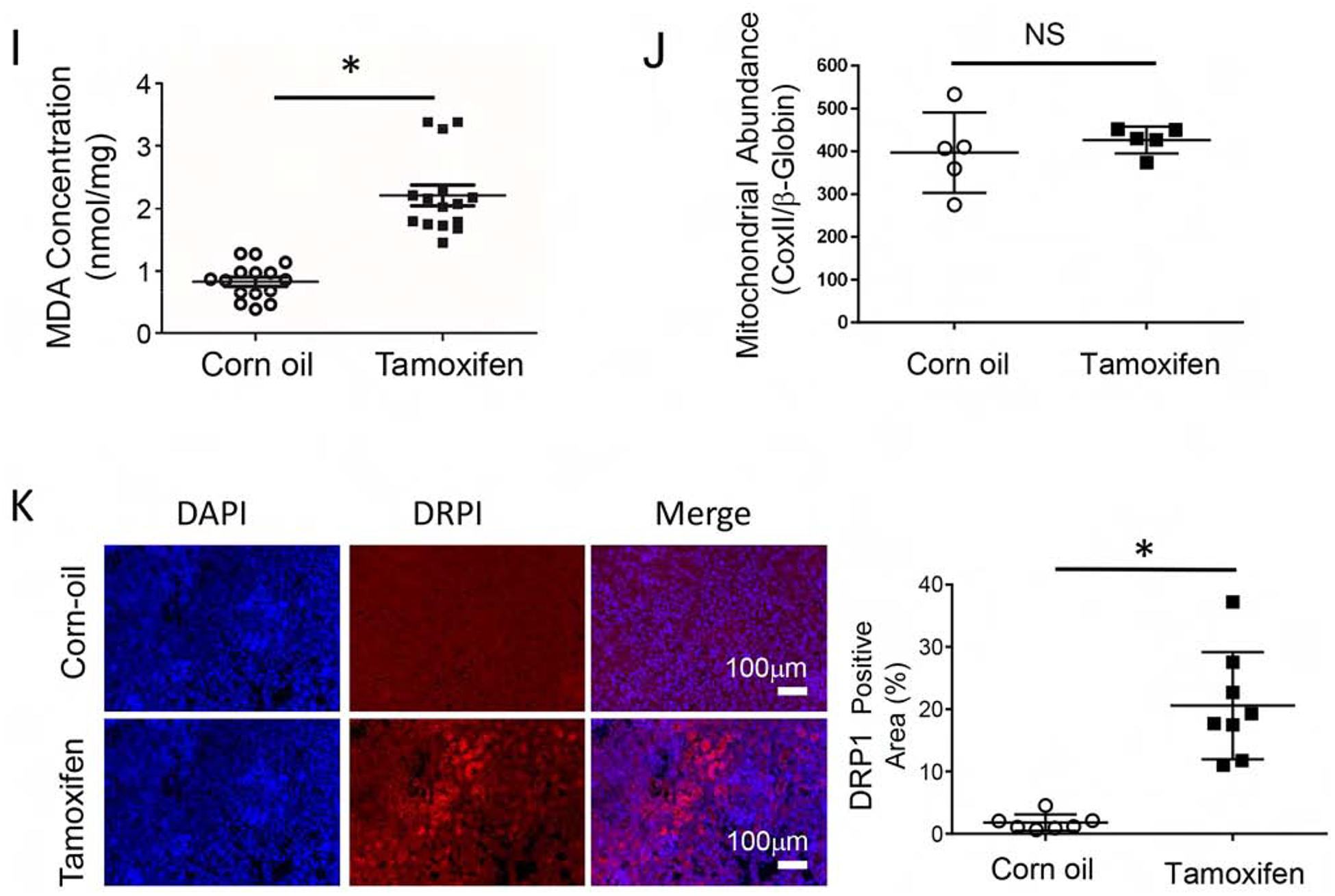
(A) Primary renal tubule epithelial cells (RTE) were isolated from 3-week-old KMDAKT mice and enrichment was quantified using the renal epithelial cell marker AQP1. (B) Primary RTE cells were treated with DMSO or 10ng/ml of tamoxifen (4-OH TAM) for transgene induction. (C) RTE cells were plated for Seahorse XF Analyzer. After basal extracellular respiration rates (OCR) analysis, different inhibitors were injected sequentially to measure different stages of respiration (complex V inhibitor: oligomycin, uncoupler: carbonyl cyanide 4-(trifluoromethoxy) phenylhydrazone, and complex I inhibitor: Rotenone) as shown in D–G. The basal respiration, (p<0.001), spare respiration (p=0.00076), ATP dependent respiration (p<0.001) and proton leak (p<0.001) were all significantly higher in the 4-OH TAM treated cells than DMSO treated cells. (H) Sixty hours after 4-OH TAM or DMSO treatment, ATP levels were reduced in KMDAKT cells expressing dnAKT1 (*p<0.04). (I) Lipid peroxidation in KMDAKT kidneys. Lipid peroxidation was measured and the results showed higher lipid peroxidation in the Tam-KMDAKT kidneys. *p < 0.01. (J) Abundance of mitochondria in KMDAKT kidneys. The content of mitochondria DNA was analyzed by real-time PCR and presented as a ratio of mitochondria DNA to nuclear DNA. NS: p value not significant. (K) DRP1 staining in KMDAKT kidneys. Mitochondria fission marker DRP1 was analyzed with immunofluorescence. The bar graph summarized the results from each mice. *p< 0.001.
