Abstract
High lead (Pb) concentration in soils is becoming a severe threat to human health. It also deteriorates plants, growth, yield and quality of food. Although the use of plant growth-promoting rhizobacteria (PGPR), biochar and compost can be effective environment-friendly amendments for decreasing Pb stress in crop plants, the impacts of their simultaneous co-application has not been well documented. Thus current study was carried, was conducted to investigate the role of rhizobacteria and compost mixed biochar (CB) under Pb stress on selected soil properties and agronomic parameters in mint (Mentha piperita L.) plants. To this end, six treatments were studied: Alcaligenes faecalis, Bacillus amyloliquefaciens, CB, PGPR1 + CB, PGPR2 + CB and control. Results showed that the application A. faecalis + CB significantly decreased soil pH and EC over control. However, OM, nitrogen, phosphorus and potassium concentration were significantly improved in the soil where A. faecalis + CB was applied over control. The A. faecalis + CB treatment significantly improved mint plant root dry weight (58%), leaves dry weight (32%), chlorophyll (37%), and N (46%), P (39%) and K (63%) leave concentration, while also decreasing the leaves Pb uptake by 13.5% when compared to the unamended control. In conclusion, A. faecalis + CB has a greater potential to improve overall soil quality, fertility and mint plant productivity under high Pb soil concentration compared to the sole application of CB and A. faecalis.
Subject terms: Microbiology techniques, Plant symbiosis, Soil microbiology, Pollution remediation
Introduction
Heavy metals are potential toxic for humans, animals, soil microorganisms and plants1–6. Presence of heavy metals beyond the threshold limit in soils adversely affects crop productivity7–9. Among others, lead (Pb) has become one of the major soil contaminants that continuously deteriorate soil health10,11. Although a non-essential nutrients for plant growth, Pb in solution can relatively easily be taken up by plants in their natural environment and accumulate as an insoluble form within plants roots12. Once inside the plant, Pb disturbs a broad range of biochemical and physiological metabolic processes including nitrate assimilation, water status plant growth and seed germination, and this results in poor growth and development13–16. However, Pb translocation is limited in plants and mostly occurs from roots to shoot but not the inverse way17. Other physiological and biochemical attributes such as carotenoid content and activity, CO2 assimilation rate, and chlorophyll and photosynthetic rate, among others, are significantly decreased under over-optimum Pb concentrations in plant13. It is well documented that fertilizers and automobiles are major sources of Pb pollution generation18. Other anthropogenic activities such as i.e., burning of fossil fuels also facilitate the overaccumulation of Pb in water, air, and soil19. However, weathering of Pb enriched rocks, use of sewage water for irrigation purposes, shedding paint chips, use of leaded gasoline in motor vehicles and waste disposal represent additional sources of Pb accumulation in soil20.
Over the last 20 years, scientists around the world have investigated and further refined different strategies to reduce the Pb toxicity problem in crops1,21,22. Among these, biochar application has been reported as one of the most promising to ameliorate the toxic effects of high heavy metals concentrations in soils and crops23. Specific properties of biochar like high surface area, porosity and adsorption rates are largely dependent on the pyrolysis conditions and feedstock type, and can confer biochar with the ability to retain and sorb numerous compounds in the soil such as organic contaminants and heavy metals23–29. Additionally, the exchange capacity, microporous structure and active functional groups of biochar played a vital role in minimizing the bioavailability and mobilization of heavy metals30.
Use of organic amendments31,32 i.e., compost in agriculture increases rhizobacterial proliferation, water and nutrients holding capacity and soil aggregation. It also decreases soil pH when applied in soil33. By maintaining the soil organic pool, compost in soil enhances the phytoavailability of macro and micronutrients by improving soil health33–35, although foliar application of fertilizer and micronutrients as demonstrated to be a better alternative to fast action in some cases34–38. Several rhizobacteria have also been identified as potential biofertilizers that could have positive effects on crop quality and yield37–45. These rhizobacteria are found in the plant rhizosphere and are collectively known as plant growth-promoting rhizobacteria (PGPR)44–48. Most PGPR secretes phytohormones, mobilized nutrients in the soil, nonsymbiotically fixed nitrogen, and decreased stress ethylene in crops46,49. Additionally, these PGPR also enhanced systemic resistance (disease-resistance mechanisms) and proved biocontrol agents against diseases49,50. Plant growth-promoting bacteria (PGPB) help in the mitigation of abiotic stresses in plants51,52. Besides the profound positive impacts that use of biostimulants have shown in overall soil health and fertility53,54, use of biostimulants can also help to reduce the phytotoxicity resulting from high soil Pb concentrations55.
Mint (Mentha piperita L.), a plant that belongs to Labiatae family, is cultivated under both field and greenhouse conditions in Pakistan56 for the production of fresh or dried herbs and essential oils57. Fresh and dried mint herbs for flavoring of beverages and foods and used for teas. Mint essential oils are used on a large scale as aromatic agents in toothpaste, chewing gum, mouthwash, candy, and aromatherapy. Mint essential oils are also used in eco-friendly pesticides, antimicrobial agents and pharmaceuticals58. The essential oils, extracts and herbs contain a big history of medicinal usage for symptomatic and therapy treatments of numerous human disorders and diseases59. However, mint plants are particularly susceptible to high concentrations of heavy metals in soils, particularly Pb60, a heavy metal that has been reported in high concentration across several soils and ecosystems in Pakistan, according to The World Health Organization61. Therefore, a pot experiment was conducted to investigate the impacts of co-application of rhizobacteria in the presence and absence of mixed biochar (CB) on growth and Pb uptake in mint. It is hypothesized that co-application of rhizobacteria and CB could be an efficacious technique for alleviation of Pb toxicity in mint over sole application.
Results
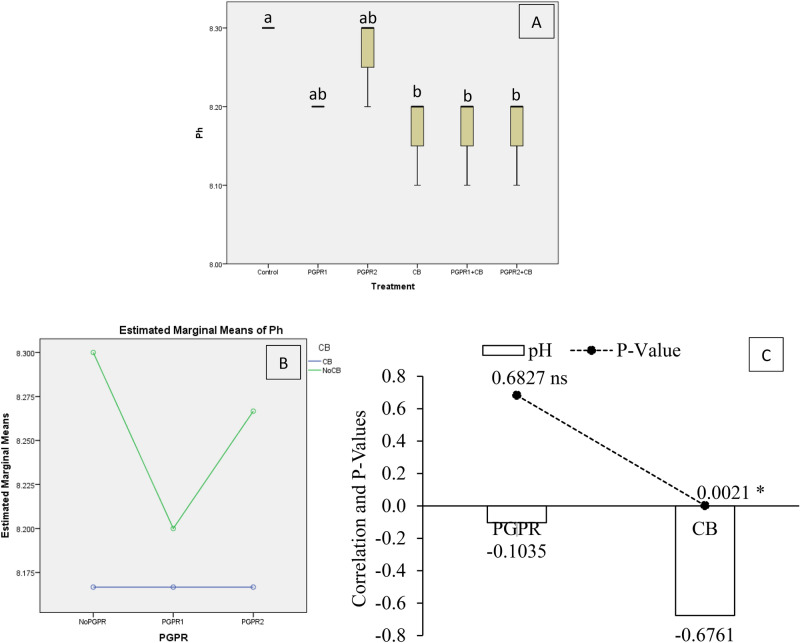
The effect of treatments was significant on soil pH under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for soil pH (Fig. 1B). Results showed that PGPR1 + CB, CB, and PGPR2 + CB significantly decreased soil pH over control (Fig. 1A). No significant increase was noted over soil pH of control, where sole inoculation of PGPR1 and PGPR2 was done. It was noted that CB showed significant (0.0021) negative (− 0.6761) correlation while PGPR showed non-significant (0.6821) negative (− 0.1035) correlation with soil pH (Fig. 1C). A significant reduction of 1.63% in soil pH was observed over control in PGPR2 + CB, CB and PGPR1 + CB.
Figure 1.
Effect of treatments on pH of soil. Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for soil pHs (B). Correlation graph of PGPR and CB for soil pHs (C).
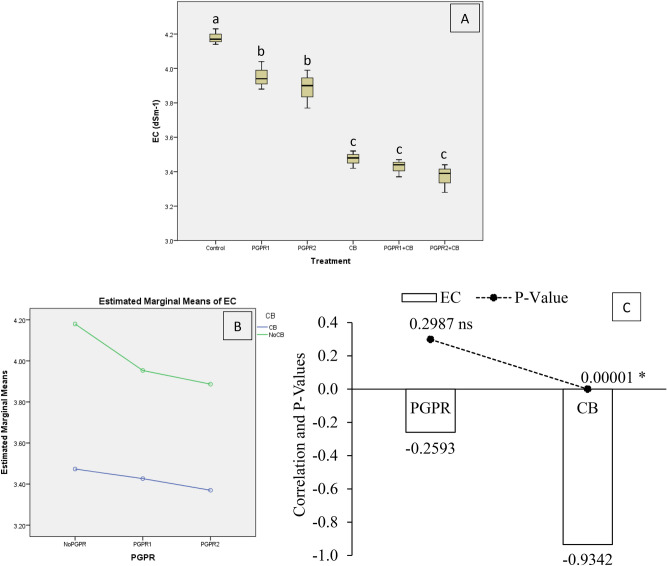
Soil EC was significantly affected by treatments under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for soil EC (Fig. 2B). Results showed that PGPR1 + CB, CB, and PGPR2 + CB were significantly decreased soil EC over soil EC of control (Fig. 2A). A significant decrease in soil EC was also noted in PGPR1 and PGPR2 over control. Application of CB remained significantly better over PGPR for decreasing soil EC as compared to control. However, PGPR1 + CB, CB and PGPR2 + CB were non-significantly with each other for soil EC. It was noted that CB showed a significant (0.00001) negative (− 0.9342) correlation, while PGPR showed a non-significant (0.2987) negative (− 0.2593) correlation with soil EC (Fig. 2C). A significant reduction of 24% in soil EC was observed in over control, where PGPR2 + CB was applied.
Figure 2.
Effect of treatments on EC of soil. Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for soil EC (B). Correlation graph of PGPR and CB for soil EC (C).
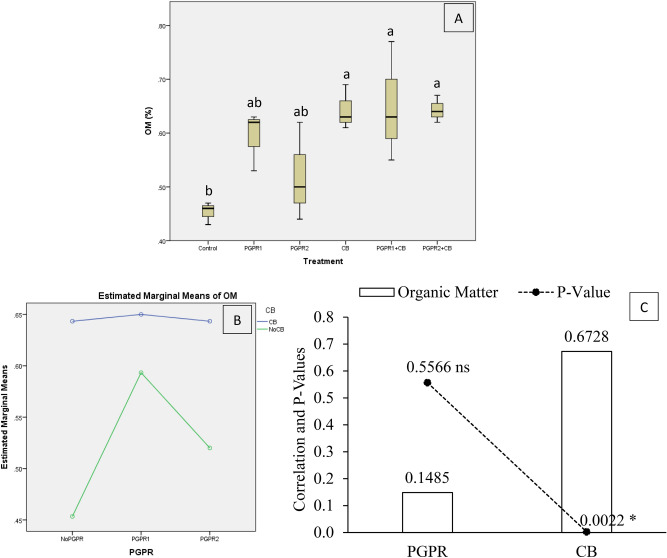
Results showed that the treatment's effect was significant on soil organic matter (OM) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for OM (Fig. 3B). Results showed that CB, PGPR1 + CB and PGPR2 + CB significantly enhanced soil OM over control (Fig. 3A). Sole inoculation of PGPR1 and PGPR2 showed neither a significant increase nor decreased soil OM over control. Application of CB remained significantly better over PGPR1 and PGPR2 for improving the OM over control. However, CB, PGPR1 + CB and PGPR2 + CB did not differ significantly from each other for OM. It was noted that CB showed significant (0.0022) positive (0.6728) correlation while PGPR showed non-significant (0.5566) positive (0.1485) correlation with OM (Fig. 3C). A significant increase of 44% in soil OM was observed in over control where PGPR1 + CB was applied.
Figure 3.
Effect of treatments on the organic matter of the soil. Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for soil OM (B). Correlation graph of PGPR and CB for soil OM (C).
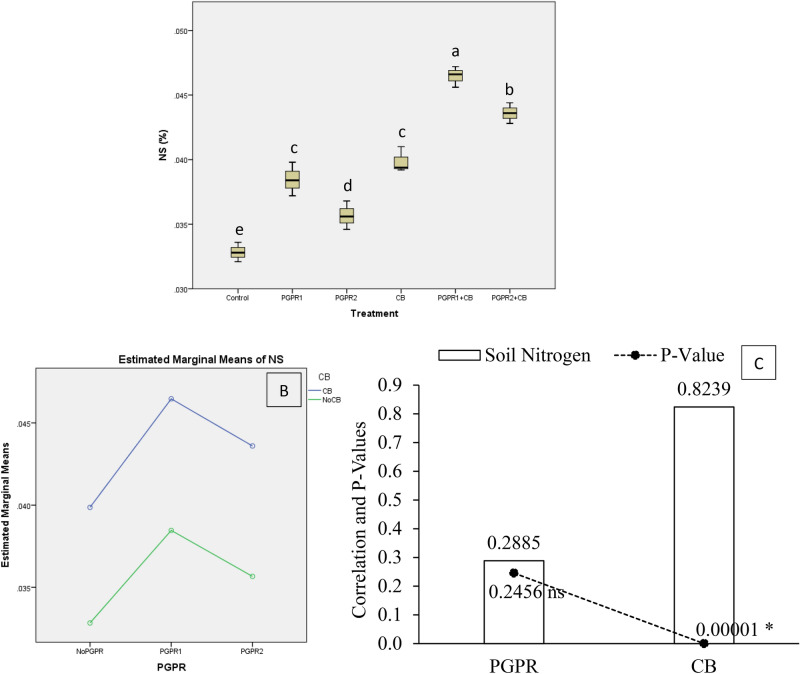
All the treatments significantly affect soil nitrogen (NS) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for NS (Fig. 4B). Results showed that PGPR1 + CB significantly enhanced NS over control (Fig. 4A). Application of PGPR2 + CB also gave significantly higher NS over CB and control. Treatments PGPR1 and PGPR2 differed significantly over control for NS. Application of CB remained significantly better over PGPR2 but statistically alike with PGPR1 for improving the NS over control. It was noted that CB showed significant (0.00001) positive (0.8239) correlation while PGPR showed non-significant (0.2456) positive (0.2885) correlation with NS (Fig. 4C). A significant increase of 42% in NS was observed in over control, where PGPR1 + CB was applied.
Figure 4.
Effect of treatments on soil nitrogen (NS). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for soil NS (B). Correlation graph of PGPR and CB for soil NS (C).
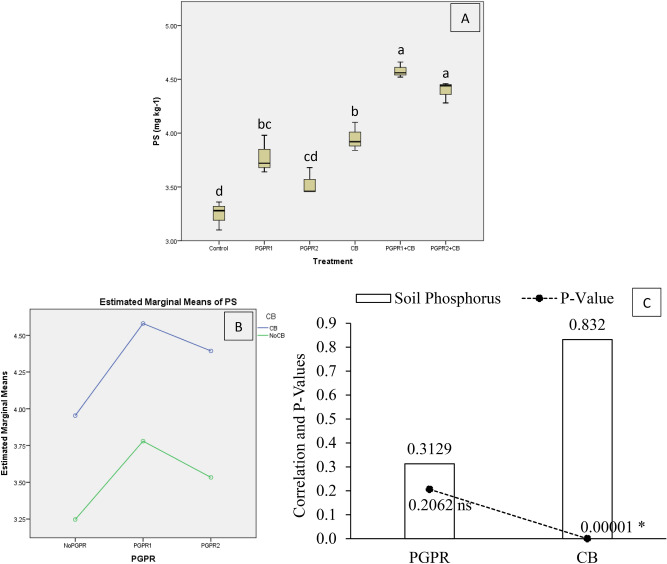
Results indicated that treatments significantly affect soil phosphorus (PS) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but disordinal for soil PS (Fig. 5B). Treatments PGPR1 + CB and PGPR2 + CB were significant over control for PS (Fig. 5A). Application of PGPR1 and CB significantly increased PS over control. Treatments PGPR1 gave significantly high PS, but PGPR2 remained non-significant over control. It was noted that CB showed significant (0.00001) positive (0.8320) correlation while PGPR showed non-significant (0.2062) positive (0.3129) correlation with PS (Fig. 5C). A significant increase of 41% in PS was observed in over control, where PGPR1 + CB was applied.
Figure 5.
Effect of treatments on soil phosphorus (PS). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for soil PS (B). Correlation graph of PGPR and CB for soil PS (C).
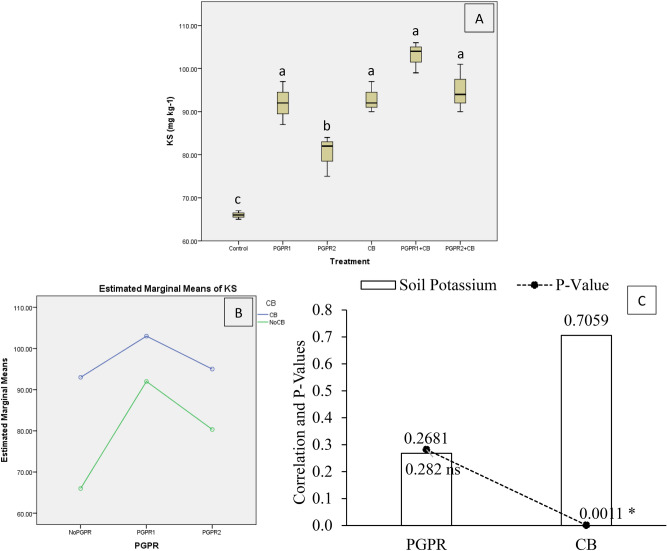
Results indicated that treatments significantly affect soil potassium (KS) under artificially induced lead (Pb) stress. Interaction of CB with PGPR was significant ordinal for KS (Fig. 6B). Application of PGPR1, PGPR1 + CB, CB and PGPR2 + CB were non-significant with each other but gave a significant increase in KS than control (Fig. 6A). Treatment PGPR2 also showed significantly high KS over control. It was noted that CB showed significant (0.0011) positive (0.7059) correlation while PGPR showed non-significant (0.2820) positive (0.2681) correlation with KS (Fig. 6C). A significant increase of 56% in KS was observed in over control, where PGPR1 + CB was applied.
Figure 6.
Effect of treatments on soil potassium (KS). Values are the average of three replicates (A). Different letters showed significant differences (Tukey ‘s test; p ≤ 0.05). Interaction graph of PGPR and CB for soil KS (B). Correlation graph of PGPR and CB for soil KS (C).
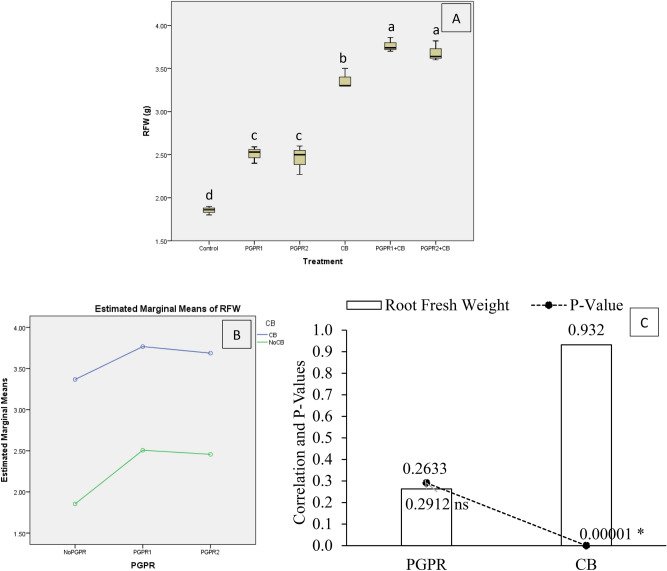
The effect of treatments was significant on mint plants root fresh weight (RFW) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for soil RFW (Fig. 7B). Treatments PGPR1 + CB and PGPR2 + CB significantly enhanced RFW over control (Fig. 7A). Sole application of CB gave significantly high RFW from control. Inoculation of PGPR1 and PGPR2 also showed a significant increase in RFW over control. It was noted that CB showed significant (0.00001) positive (0.9320) correlation while PGPR showed non-significant (0.2912) positive (0.2633) correlation with RFW (Fig. 7C). A significant increase of 1.03-fold in RFW was observed in over control where PGPR1 + CB was applied.
Figure 7.
Effect of treatments on mint plants roots fresh weight (RFW). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for RFW (B). Correlation graph of PGPR and CB for RFW (C).
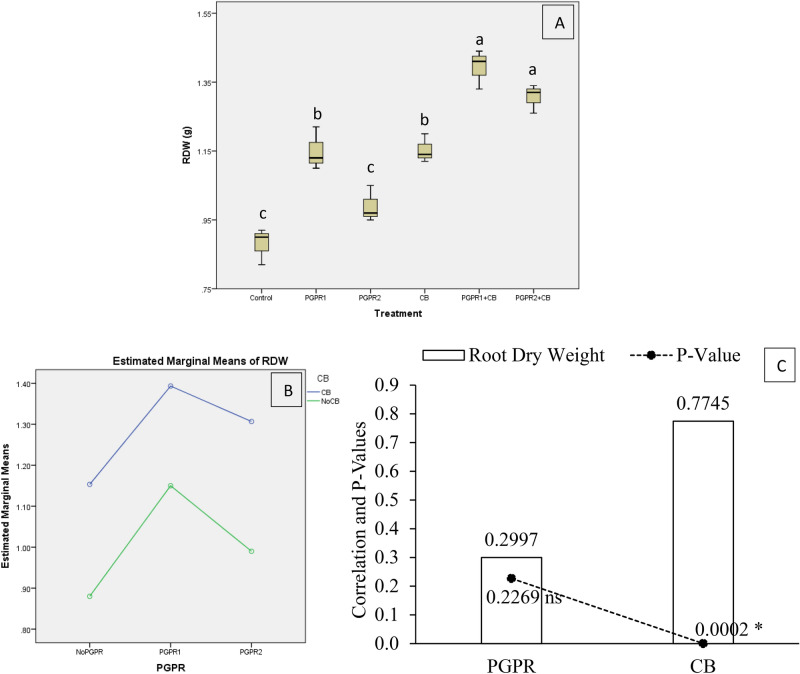
Application of treatments significantly affects the mint plant's root dry weight (RDW) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for RDW (Fig. 8B). Treatments PGPR1 + CB and PGPR2 + CB gave a significant increase in RDW over control (Fig. 8A). Treatments PGPR2 was non-significant over control for RDW. Inoculation of PGPR1 and CB application remained statistically alike but gave a significant increase in RDW over control. It was noted that CB showed significant (0.0002) positive (0.7745) correlation while PGPR showed non-significant (0.2269) positive (0.2997) correlation with RDW (Fig. 8C). A significant increase of 58% in RDW was observed in over control, where PGPR1 + CB was applied.
Figure 8.
Effect of treatments on mint plants root dry weight (RDW). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for RDW (B). Correlation graph of PGPR and CB for RDW (C).
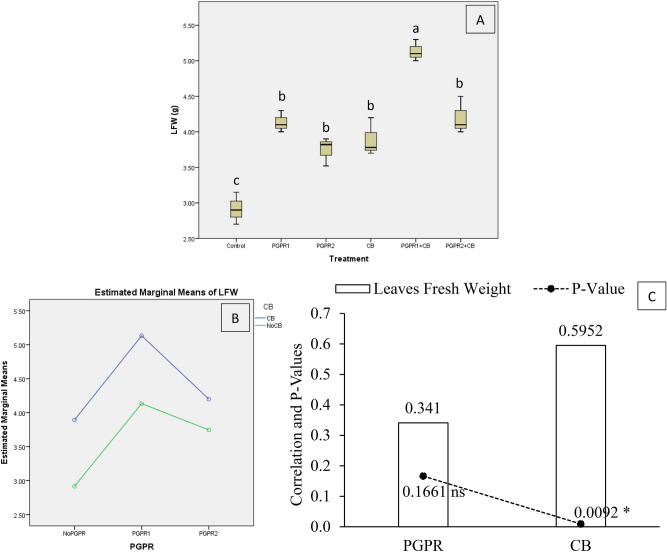
The addition of treatments significantly affects mint leaves fresh weight (LFW) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for LFW (Fig. 9B). Treatments PGPR1 + CB significantly increased LFW over control (Fig. 9A). Application of PGPR1, PGPR2, CB and PGPR2 + CB gave significant enhancement in LFW over control. It was noted that CB showed significant (0.0092) positive (0.5952) correlation while PGPR showed non-significant (0.1661) positive (0.3410) correlation with LFW (Fig. 9C). A significant increase of 76% in LFW was observed in over control, where PGPR1 + CB was applied.
Figure 9.
Effect of treatments on mint plants leaves fresh weight (LFW). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for LFW (B). Correlation graph of PGPR and CB for LFW (C).
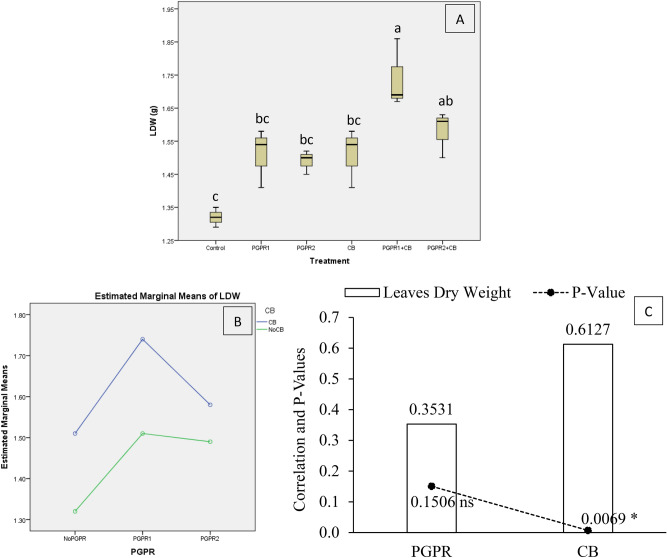
Results showed that treatment's application remained significantly different for mint leaves dry weight (LDW) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for LDW (Fig. 10B). Treatments PGPR1 + CB and PGPR2 + CB remained statistically alike but increased LDW significantly over control (Fig. 10A). Inoculation of PGPR1, PGPR2 and CB remained non-significant over control for LDW. It was noted that CB showed significant (0.0069) positive (0.6127) correlation while PGPR showed non-significant (0.1506) positive (0.3531) correlation with LDW (Fig. 10C). A significant increase of 32% in LDW was observed in over control where PGPR1 + CB was applied.
Figure 10.
Effect of treatments on mint plants leaves dry weight (LDW). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for LDW (B). Correlation graph of PGPR and CB for LDW (C).
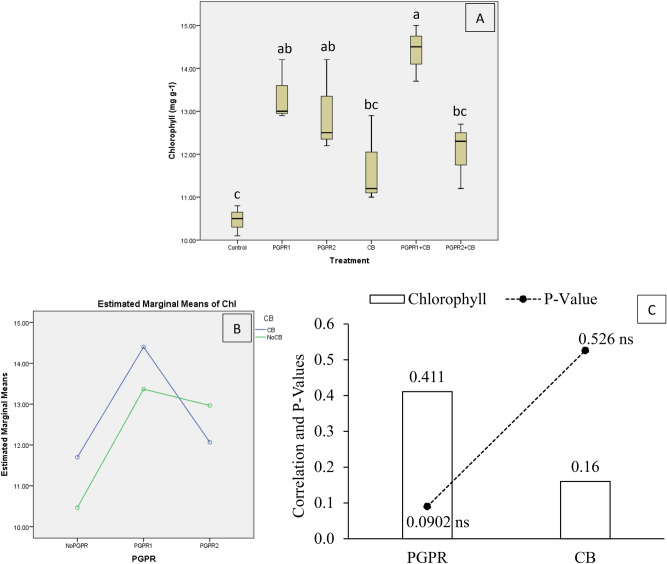
Results showed that treatment's application remained significantly different for mint chlorophyll contents (Chl) under artificially induced lead (Pb) stress. Interaction of PGPR and CB was significant ordinal for Chl (Fig. 11B). Treatments PGPR1 + CB, PGPR1 and PGPR2 increase Chl significantly over control (Fig. 11A). Application of PGPR2 + CB and CB remained non-significant over control for Chl. It was noted that CB showed non-significant (0.5260) positive (0.1600) correlation while PGPR showed non-significant (0.0902) positive (0.4110) correlation with Chl (Fig. 11C). A significant increase of 37% in Chl was observed in over control, where PGPR1 + CB was applied.
Figure 11.
Effect of treatments on mint plants leaves chlorophyll contents. Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for Chl (B). Correlation graph of PGPR and CB for Chl (C).
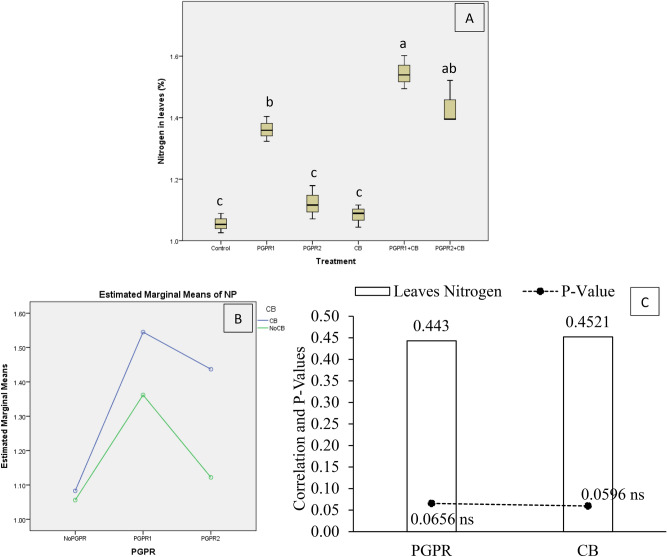
Nitrogen concentration in mint leaves (NP) was significantly affected by treatments under artificially induced lead (Pb) stress. Interaction of PGPR and CB was significant ordinal for NP (Fig. 12B). Treatments PGPR1 + CB and PGPR2 + CB remained statistically alike but significantly increase NP over control (Fig. 12A). Application of CB and PGPR2 remained non-significant over control for NP. However, sole inoculation of PGPR1 gave a significant increase in NP over control. It was noted that CB showed non-significant (0.0596) positive (0.4521) correlation while PGPR showed non-significant (0.0656) positive (0.4430) correlation with NP (Fig. 12C). A significant increase of 46% in NP was observed in over control, where PGPR1 + CB was applied.
Figure 12.
Effect of treatments on mint plants leaves nitrogen concentration (NP). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for NP (B). Correlation graph of PGPR and CB for NP (C).
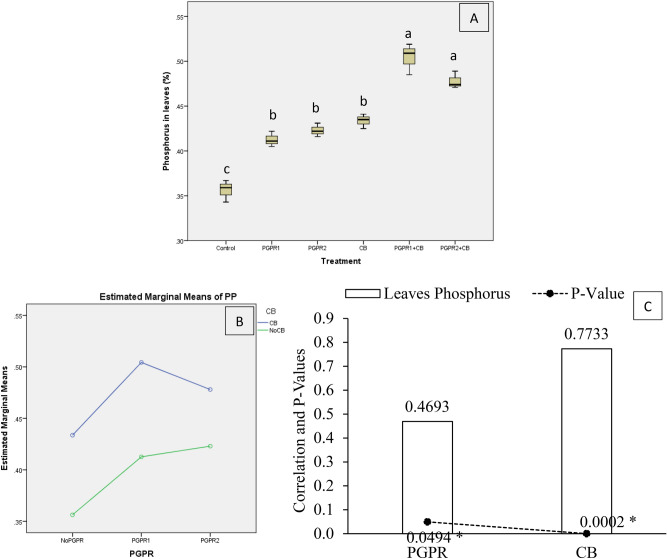
Phosphorus concentration in mint leaves (PP) was significantly affected by treatments under artificially induced lead (Pb) stress. Interaction of PGPR and CB was significant ordinal for PP (Fig. 13B). The application of PGPR1 + CB and PGPR2 + CB remained non-significant but increased PP significantly over control (Fig. 13A). However, treatments PGPR1, PGPR2 and application of CB also remained significant for PP over control. It was noted that CB showed significant (0.0002) positive (0.7733) correlation while PGPR also showed significant (0.0494) positive (0.4693) correlation with PP (Fig. 13C). A significant increase of 39% in PP was observed in over control, where PGPR1 + CB was applied.
Figure 13.
Effect of treatments on mint plants leaves phosphorus concentration (PP). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for soil PP (B). Correlation graph of PGPR and CB for soil PP (C).
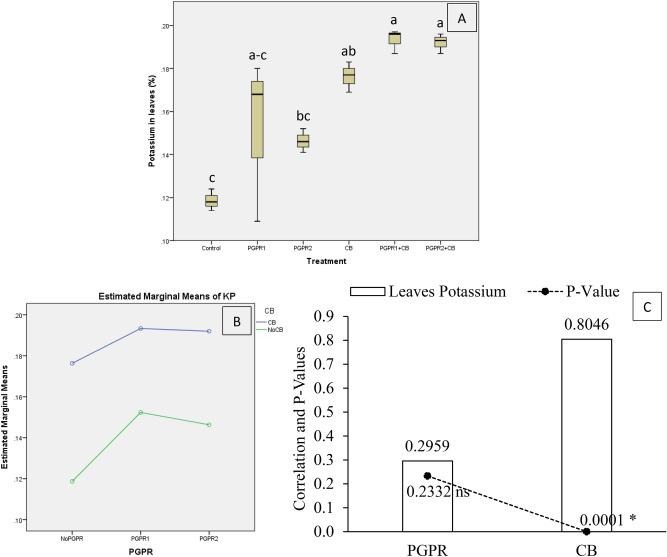
Potassium concentration in mint leaves (KP) was significantly affected by treatments under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for KP (Fig. 14B). Treatments PGPR1 + CB, CB and PGPR2 + CB, increased KP significantly over control (Fig. 14A). However, treatments PGPR2 and PGPR1 also remained non-significant for KP over control. It was noted that CB showed significant (0.0001) positive (0.8046) correlation while PGPR also showed non-significant (0.2332) positive (0.2959) correlation with KP (Fig. 14C). A significant increase of 63% in KP was observed in over control, where PGPR1 + CB was applied.
Figure 14.
Effect of treatments on mint plants leaves potassium concentration (KP). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for KP (B). Correlation graph of PGPR and CB for KP (C).
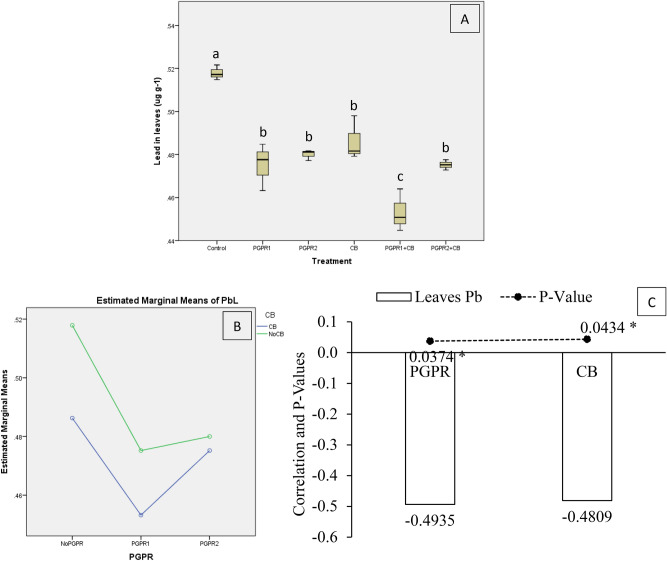
Lead concentration in mint leaves (PbL) was significantly affected by treatments under artificially induced lead (Pb) stress. Interaction of PGPR and CB was non-significant but ordinal for PbL. Application of PGPR1 + CB significantly decreased PbL over control (Fig. 15A,B). However, sole inoculation of PGPR2, PGPR1, CB and PGPR2 + CB decreased PbL significantly over control. It was noted that CB showed a significant (0.0434) negative (− 0.4809) correlation, while PGPR also showed a significant (0.0374) negative (− 0.4935) correlation with PbL (Fig. 15C). A significant decrease of 13.5% in PbL was observed in over control, where PGPR1 + CB was applied.
Figure 15.
Effect of treatments on mint plants leaves lead concentration (PbL). Values are the average of three replicates (A). Different letters showed significant differences (Tukey’s test; p ≤ 0.05). Interaction graph of PGPR and CB for PbL (B). Correlation graph of PGPR and CB for PbL (C).
Discussion
In the current study, the application of CB with and without PGPR decreased soil pH. This decrease in soil pH was due to the low pH of compost and biochar over the soil. Also, the secretion of organic acids by rhizobacteria played an imperative role in decreasing soil pH. Most of the chemical properties of biochar are dependent on the nature of feedstock26,62. Combined application of organic amendments and biochar catalyzed the oxidation processes63. A significant enhancement in biochar oxidation played an efficacious role in releasing the carboxylic functional group in soil. This carboxylic functional group decrease soil pH thus, regulate the exchange of mineralized ions64,65. Many rhizobacteria in soil secrete organic acids, which also act as an allied factor for decreasing soil pH66. Besides biochar, the decomposition of compost in the soil also releases acidic compounds67. The presence of a high concentration of humic acid in composted materials also efficaciously played its role in decreasing soil pH68.
Furthermore, compost has readily degradable carbon compounds by microbes of the rhizosphere by acidic secretions. These compounds are soluble in water and contribute to the decrease of soil pH69. Biochar has the potential to enhance the soil ions exchangeability70.
Enhance cation exchange capacity, in turn, increases ion retention in the rhizosphere. As a result, ECe of the soil is increased71. Furthermore, colonization of PGPRs enhances the root's surface area, which facilitates the plants regarding better nutrients uptake72. Under partial or no supply of oxygen and high-temperature combustion caused carbon sequestration, which yields activated carbon (biochar)62. Better soil aggregation after biochar addition facilitates soil OM buildup71–75. It also enhances soil microbial proliferation and activities in the rhizosphere76,77. Also, the use of compost in the current study was an allied factor for a significant increase in soil organic matter. Continuous addition of compost as amendments significantly improves soil organic matter on a long-term basis78. Besides, biochar can also control soil nutrients losses by leaching79. Better retention of soil nutrients due to the high exchangeability of biochar improves soil's fertility status80,81. PGPR secrets different organic acids (tartaric acid, oxalic acid, malic acid, citric acid, succinic acid) that modify soil pH. Siderophores produced by PGPR actively chelate potassium ions and enhances their bioavailability to the crops82,83.
A major part of compost also contributes to the provision of mineralized K that is an allied factor for improving soil health and fertility level84. When applied in combination with biochar, organic manure modify the plant's roots physiology, facilitating better nutrient availability85. Growth hormones, i.e., indole acetic acid (IAA) secretion by PGPR, also enhance roots elongation86. The results of the current study also support the above findings. Both rhizobacteria used in the current study were capable of producing IAA growth hormone that played an imperative role in significant plant growth improvement. Mohite87 suggested that IAA increases the growth of adventitious roots. These roots are directly involved in nutrients uptake. Compost and biochar addition in the soil thus ameliorate soil properties and increase soil fertility level linked with significant improvement in biomass production of crops88,89. Danish and Zafar-ul-Hye42 also noted the efficiency of rhizobacteria is increased when inoculated with biochar43. A significant improvement in N, P and K concentration of mint leaves also validated such results. Pore spaces and exchange sites of compost mixed biochar in the current study effectively enhanced the bioavailability of nutrients to the mint plants. In addition to the above, a significant decrease in soil pH also played a vital in the mobilization of fixed P. High contents of K in compost has also contributed to improved soil pH regarding enhancement in the K uptake in mint plants. Accumulation of stress generating ethylene in plants under toxicity of heavy metals also deteriorates crops' growth and yield. This ethylene is decomposed into α-ketobutyrate and ammonia by ACC deaminase produced by PGPRs, resulting in alleviation of stress induced by heavy metals90,91. Zafar-ul-Hye et al.41 also documented similar findings by using ACC deaminase producing PGPR under heavy metal toxicity. The compost application also helped in the provision of energy to PGPRs and enhance oxygen transfer, which facilitates the immobilization of metallic ions in soil92. As both rhizobacteria of the current study were also capable of producing ACC deaminase, they also act as an allied factor for the improvement in mint growth under Pb toxicity. Song and Greenway93 also observed that heavy metals become bounded with the compost's exchange sites in the soil. The presence of surface-active function in biochar sorp the heavy metals electrostatically thus caused their immobilization in soil94. Among different functional groups for heavy metals, immobilization through biochar CO3−2 and hydroxides are predominant95,96. Change in redox potential and rhizosphere acidification via PGPRs secretions, the bioavailability of heavy metals to plants is also decreased53,55,97,98.
Conclusion
It is concluded that both CB and A. faecalis treatments effectively minimize the Pb toxicity in min Plants. However, the use of A. faecalis + CB as a treatment is a better approach than the sole application of CB and A. faecalis under Pb toxicity for improvement in growth attributes, nutrients concentration and mitigation of Pb toxicity in mint. More investigations are suggested to introduce A. faecalis + CB as an efficient treatment for alleviating Pb stress in the mint at field levels.
Materials and methodology
Treatments preparation
From Sabzi Mandi, Multan, fruit and vegetable waste were collected for the manufacturing of biochar. To achieve < 15% moisture sun-drying of waste material was done for 14 days. After sun drying, small pieces of waste material were put in pyrolyzer at the temperature of 450 °C and pyrolyzed for 2 h under the partial oxygen presence. After that pyrolyzer drum was left for cooling. Finally, biochar was grinded and pass through 2 mm sieve. To make organic amendment (compost mixed biochar), compost was purchase from Buraq Agro Chemicals, Industrial State Area, Multan. For experimental purposes, biochar was mixed with compost in 1:1 ratio and applied in the soil at the rate of 0.5% (5 g kg−1). Application of compost mixed biochar was done at the time of pot filling with soil as per treatment plan. PGPRs i.e., Alcaligenes faecalis and Bacillus amyloliquefaciens were collected from Soil Microbiology and Biochemistry Laboratory, BZU, Multan and propagated in Dworkin and Foster (DF) media99. The inoculation of mint seeds was done using inoculum 0.5 nm optical density of inoculum (5 ml 100 g−1 seeds). The final top dressing was done with sterilized peat, clay and sugar solution (10%). Inoculation of PGPR was done before 30 min of sowing.
Experimental organization
A pot study was carried on the experimental farm of the Faculty of Agricultural Sciences and Technology. Table 1 has a pre-experimental soil characterization.
Table 1.
Analyses of compost, soil and rhizobacteria (Pre-sowing)55.
| Characteristics | Soil | Compost | Biochar | Characteristics | A. faecalis | B. amyloliquefaciens |
|---|---|---|---|---|---|---|
| Textural class | Loam | – | – | IAA L-Tryptophan (µgml−1) | 15.33 | 22.23 |
| pHs | 8.30 | 5.3 | 8.04 | |||
| ECe (dS m−1) | 1.25 | – | 3.49 | |||
| OM (%) | 0.30 | – | – | IAA (No L-Tryptophan (µg ml−1) | 2.21 | 5.63 |
| Total N (%) | 0.015 | 1.00 | 1.63 | |||
| Available phosphorus (µg g−1) | 4.62 | 0.53 | 0.40 | |||
| Extractable potassium (µg g−1) | 70 | 55 | 27 |
ACC deaminase α-ketobutyrate nmol g−1protein h−1 Exopolysaccharide |
484 | 232 |
| Extractable lead (µg g−1) | 0.50 | 1.15 | 2.09 | |||
| Volatile matter (%) | – | – | 14.4 | |||
| Ash content (%) | – | – | 16.8 | + | + | |
| Fixed carbon (%) | – | – | 68.8 | Phosphate solubilization | + | + |
Treatments
Total six treatments were applied in 3 replications following a complete randomized design (CRD). The treatments were controlled, PGPR1 (A. faecalis), PGPR2 (B. amyloliquefaciens), 1:1 compost mixed biochar (CB), PGPR1 + CB and PGPR2 + CB. Each pot was filled with 7 kg of soil, and 15 seeds of mint were sown. After germination, only five seedlings were maintained by thinning. Macronutrients were applied at the rate of 33 (K), 80 (P), and 130 (N) kg ha−1, in the form of sulphate of potash, Nitrophos and Calcium Ammonium Nitrate at the time of pot preparation. After one week of germination and thinning, Pb stress was applied artificially. Lead sulphate (PbSO4) was applied at 250 mg kg−1 soil for inducing lead stress100.
Data collection
Soil analyses
Bouyoucos hydrometer was used for the determination of soil textural class101. The pH of saturated paste was determined pre-calibrated pH meter. Electrical conductivity (EC) was assessed on a pre-calibrated EC meter. Walkley and Black102 method was used for soil organic matter determination. Olsen extraction method was adopted for the determination of soil extractable P103. Ammonium acetate was used to extract soil potassium, and K was assessed using a flame photometer104.
Chlorophyll contents
Fresh leaves were taken and cut into small pieces, and 0.5 g leaf samples were immersed in 10 ml acetone for 24 h. Extract of chlorophyll was measured, and color intensity was determined at 645 nm and 663 nm by spectrophotometer105. From intensity values, chlorophyll contents were determined by the following formula:
where OD = Optical density (wavelength), V = Final volume made, W = Fresh leaf made (g).
Plant analyses
Nitrogen was analyzed on Kjeldhals distillation apparatus106. For phosphorus determination, the plant samples were digested in an acid mixture of HNO3 and HCIO4107. The phosphorus was determined by the yellow color method at 470 nm wavelength by using spectrophotometer106. For the determination of potassium, the digested sample aliquot was fed to the flamephotometer104. The reading of di-acid digested filtrate was noted on atomic absorption spectrophotometer for determination of Pb in leaves27.
Statistical analyses
Analysis of variance was done using SPSS 20, Duncan's (p ≤ 0.05) test was applied to compare means among the different groups. Data were analyzed using the standard statistical procedure as followed by Steel et al.108.
Author contributions
M.Z.-u-H. and M.T.-u-H. Designed and conducted the experiment; S.D. helped in manuscript writing, statistical analyses; S.F. M.J.K. and A.W. helped in manuscript review; M.B. M.L.B. G.S.H. and R.D. validated statistical analyses.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Subhan Danish, Email: sd96850@gmail.com.
Shah Fahad, Email: shah_fahad80@yahoo.com.
Rahul Datta, Email: rahulmedcure@gmail.com.
References
- 1.Adrees M, et al. Mechanisms of silicon-mediated alleviation of heavy metal toxicity in plants: a review. Ecotoxicol. Environ. Saf. 2015;119:186–197. doi: 10.1016/j.ecoenv.2015.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Keller C, et al. Effect of silicon on wheat seedlings (Triticum turgidum L.) grown in hydroponics and exposed to 0 to 30 µM Cu. Planta. 2015;241:847–860. doi: 10.1007/s00425-014-2220-1. [DOI] [PubMed] [Google Scholar]
- 3.Khan AZ, et al. Popular wood and sugarcane bagasse biochars reduced uptake of chromium and lead by lettuce from mine-contaminated soil. Environ. Pollut. 2020;263:114446. doi: 10.1016/j.envpol.2020.114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zafar-Ul-hye M, et al. Alleviation of cadmium adverse effects by improving nutrients uptake in bitter gourd through cadmium tolerant rhizobacteria. Environ. MDPI. 2020;7:54. [Google Scholar]
- 5.Danish S, et al. Effect of foliar application of Fe and banana peel waste biochar on growth, chlorophyll content and accessory pigments synthesis in spinach under chromium (IV) toxicity. Open Agric. 2019;4:381–390. doi: 10.1515/opag-2019-0034. [DOI] [Google Scholar]
- 6.Zafar-ul-hye M, et al. Effect of cadmium-tolerant rhizobacteria on growth attributes and chlorophyll contents of bitter gourd under cadmium toxicity. Plants. 2020;9:1386. doi: 10.3390/plants9101386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adriano. Trace elements in terrestrial environments. Biogeochemistry, bioavailability and risks of metals. in Springer-Verlag, New York vol. 32 374 (2001).
- 8.Wang G, et al. Transfer characteristics of cadmium and lead from soil to the edible parts of six vegetable species in southeastern China. Environ. Pollut. 2006;144:127–135. doi: 10.1016/j.envpol.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 9.Sheng XF, Xia JJ, Jiang CY, He LY, Qian M. Characterization of heavy metal-resistant endophytic bacteria from rape (Brassica napus) roots and their potential in promoting the growth and lead accumulation of rape. Environ. Pollut. 2008;156:1164–1170. doi: 10.1016/j.envpol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Xia Y, et al. Efficient immobilization of toxic heavy metals in multi-contaminated agricultural soils by amino-functionalized hydrochar: Performance, plant responses and immobilization mechanisms. Environ. Pollut. 2020;261:114217. doi: 10.1016/j.envpol.2020.114217. [DOI] [PubMed] [Google Scholar]
- 11.Huang H, et al. Comparative efficacy of organic and inorganic silicon fertilizers on antioxidant response, Cd/Pb accumulation and health risk assessment in wheat (Triticum aestivum L.) Environ. Pollut. 2019;255:113146. doi: 10.1016/j.envpol.2019.113146. [DOI] [PubMed] [Google Scholar]
- 12.Wierzbicka MH, et al. Comparison of the toxicity and distribution of cadmium and lead in plant cells. Protoplasma. 2007;231:99. doi: 10.1007/s00709-006-0227-6. [DOI] [PubMed] [Google Scholar]
- 13.Seregin IV, Kozhevnikova AD. Roles of root and shoot tissues in transport and accumulation of cadmium, lead, nickel, and strontium. Russ. J. Plant Physiol. 2008;55:1–22. doi: 10.1134/S1021443708010019. [DOI] [Google Scholar]
- 14.Hockmann K, Tandy S, Studer B, Evangelou MWH, Schulin R. Plant uptake and availability of antimony, lead, copper and zinc in oxic and reduced shooting range soil. Environ. Pollut. 2018;238:255–262. doi: 10.1016/j.envpol.2018.03.014. [DOI] [PubMed] [Google Scholar]
- 15.Lamhamdi M, Bakrim A, Aarab A, Lafont R, Sayah F. Lead phytotoxicity on wheat (Triticum aestivum L.) seed germination and seedlings growth. Comptes Rendus Biol. 2011;334:118–126. doi: 10.1016/j.crvi.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 16.Uslu OS, Babur E, Alma MH, Solaiman ZM. Walnut shell biochar increases seed germination and early growth of seedlings of fodder crops. Agric. 2020;10:1–13. doi: 10.37478/agr.v10i1.75. [DOI] [Google Scholar]
- 17.Sharma P, Dubey RS. Lead toxicity in plants. Braz. J. Plant Physiol. 2005;17:35–52. doi: 10.1590/S1677-04202005000100004. [DOI] [Google Scholar]
- 18.Hall JL. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002;53:1–11. doi: 10.1093/jexbot/53.366.1. [DOI] [PubMed] [Google Scholar]
- 19.Saleem M, Asghar HN, Zahir ZA, Shahid M. Impact of lead tolerant plant growth promoting rhizobacteria on growth, physiology, antioxidant activities, yield and lead content in sunflower in lead contaminated soil. Chemosphere. 2018;195:606–614. doi: 10.1016/j.chemosphere.2017.12.117. [DOI] [PubMed] [Google Scholar]
- 20.Ehsan N, et al. Use of ornamental plant, ‘Vinca’ (Vinca rosea L.) for remediation of lead-contaminated soil. J. Biodivers. Environ. Sci. 2016;8:46–54. [Google Scholar]
- 21.Ahmad M, et al. Lead and copper immobilization in a shooting range soil using soybean stover- and pine needle-derived biochars: chemical, microbial and spectroscopic assessments. J. Hazard. Mater. 2016;301:179–186. doi: 10.1016/j.jhazmat.2015.08.029. [DOI] [PubMed] [Google Scholar]
- 22.Diatta AA, Fike JH, Battaglia ML, Galbraith JM, Baig MB. Effects of biochar on soil fertility and crop productivity in arid regions: a review. Arab. J. Geosci. 2020;13:595. doi: 10.1007/s12517-020-05586-2. [DOI] [Google Scholar]
- 23.Rizwan M, et al. Mechanisms of biochar-mediated alleviation of toxicity oftrace elements in plants: a critical review. Environ. Sci. Pollut. Res. 2016;23:2230–2248. doi: 10.1007/s11356-015-5697-7. [DOI] [PubMed] [Google Scholar]
- 24.Ok YS, Chang SX, Gao B, Chung HJ. SMART biochar technology-A shifting paradigm towards advanced materials and healthcare research. Environ. Technol. Innov. 2015;4:206–209. doi: 10.1016/j.eti.2015.08.003. [DOI] [Google Scholar]
- 25.Younis U, Danish S, Shah MHR, Malik SA. Nutrient shifts modeling in Spinacea oleracea L. and Trigonella corniculata L. in contaminated soil amended with biochar. Int. J. Biosci. 2014;5:89–98. [Google Scholar]
- 26.Qayyum MF, Abid M, Danish S, Saeed MK, Ali MA. Effects of various biochars on seed germination and carbon mineralization in an alkaline soil. Pakistan J. Agric. Sci. 2014;51:977–982. [Google Scholar]
- 27.Younis U, et al. Growth, survival, and heavy metal (Cd and Ni) uptake of spinach (Spinacia oleracea) and fenugreek (Trigonella corniculata) in a biochar-amended sewage-irrigated contaminated soil. J. Plant Nutr. Soil Sci. 2015;178:209–217. doi: 10.1002/jpln.201400325. [DOI] [Google Scholar]
- 28.Hashmi S, Younis U, Danish S, Munir TM. Pongamia pinnata L. leaves biochar increased growth and pigments syntheses in Pisum sativum L. exposed to nutritional stress. Agric. 2019;9:153. [Google Scholar]
- 29.Danish S, Younis U, Nasreen S, Akhtar N, Iqbal MT. Biochar consequences on cations and anions of sandy soil. J. Biodivers. Environ. Sci. 2015;6:121–131. [Google Scholar]
- 30.Jiang J, Xu R, Jiang T, Li Z. Immobilization of Cu(II), Pb(II) and Cd(II) by the addition of rice straw derived biochar to a simulated polluted Ultisol. J. Hazard. Mater. 2012;229–230:145–150. doi: 10.1016/j.jhazmat.2012.05.086. [DOI] [PubMed] [Google Scholar]
- 31.Izhar Shafi M, et al. Application of single superphosphate with humic acid improves the growth, yield and phosphorus uptake of wheat (Triticum aestivum L.) in calcareous soil. Agronomy. 2020;10:1224. doi: 10.3390/agronomy10091224. [DOI] [Google Scholar]
- 32.Wahid F, et al. Sustainable management with mycorrhizae and phosphate solubilizing bacteria for enhanced phosphorus uptake in calcareous soils. Agriculture. 2020;10:334. doi: 10.3390/agriculture10080334. [DOI] [Google Scholar]
- 33.Schulz H, Dunst G, Glaser B. No effect level of Co-composted biochar on plant growth and soil properties in a greenhouse experiment. Agronomy. 2014;4:34–51. doi: 10.3390/agronomy4010034. [DOI] [Google Scholar]
- 34.Doan TT, et al. Influence of buffalo manure, compost, vermicompost and biochar amendments on bacterial and viral communities in soil and adjacent aquatic systems. Appl. Soil Ecol. 2014;73:78–86. doi: 10.1016/j.apsoil.2013.08.016. [DOI] [Google Scholar]
- 35.Babur E, Dindaroğlu T, Solaiman ZM, Battaglia ML. Microbial respiration, microbial biomass and activity are highly sensitive to forest tree species and seasonal patterns in the Eastern Mediterranean Karst Ecosystems. Sci. Total Environ. 2021;775:145868. doi: 10.1016/j.scitotenv.2021.145868. [DOI] [Google Scholar]
- 36.Tariq M, et al. Effect of micronutrients foliar supplementation on the production and eminence of plum (Prunus domestica L.) Qual. Assur. Saf. Crop. Foods. 2020;12:32–40. doi: 10.15586/qas.v12iSP1.793. [DOI] [Google Scholar]
- 37.Rafiullah MJK, et al. Phosphorus nutrient management through synchronization of application methods and rates in wheat and maize crops. Plants. 2020;9:1389. doi: 10.3390/plants9101389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rafiullah, et al. Effect of micronutrients foliar supplementation on the production and eminence of plum. Qual. Assur. Saf. Crop. Foods. 2020;12:32–40. doi: 10.15586/qas.v12iSP1.793. [DOI] [Google Scholar]
- 39.Danish S, et al. Phosphorus solubilizing bacteria and rice straw biochar consequence on maize pigments synthesis. Int. J. Biosci. 2015;5:31–39. [Google Scholar]
- 40.Danish S, Zafar-ul-Hye M, Hussain M, Shaaban M, Núñez-delgado A. Rhizobacteria with ACC-deaminase activity improve nutrient uptake, chlorophyll contents and early seedling growth of wheat under PEG-induced osmotic stress. Int. J. Agric. Biol. 2019;21:1212–1220. [Google Scholar]
- 41.Zafar-ul-Hye M, Shahjahan A, Danish S, Abid M, Qayyum MF. Mitigation of cadmium toxicity induced stress in wheat by ACC-deaminase containing PGPR isolated from cadmium polluted wheat rhizosphere. Pakistan J. Bot. 2018;50:1727–1734. [Google Scholar]
- 42.Danish S, Zafar-ul-Hye M. Co-application of ACC-deaminase producing PGPR and timber-waste biochar improves pigments formation, growth and yield of wheat under drought stress. Sci. Rep. 2019;9:5999. doi: 10.1038/s41598-019-42374-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zafar-ul-Hye M, Danish S, Abbas M, Ahmad M, Munir TM. ACC deaminase producing PGPR Bacillus amyloliquefaciens and agrobacterium fabrum along with biochar improve wheat productivity under drought stress. Agronomy. 2019;9:343. doi: 10.3390/agronomy9070343. [DOI] [Google Scholar]
- 44.Zafar-ul-Hye M, Zahra MB, Danish S, Abbas M. Multi-strain inoculation with PGPR producing ACC deaminase is more effective than single-strain inoculation to improve wheat (Triticum aestivum) growth and yield. Phyton-Int. J. Exp. Bot. 2020;89:405–413. [Google Scholar]
- 45.Danish S, Zafar-ul-Hye M. Combined role of ACC deaminase producing bacteria and biochar on cereals productivity under drought. Phyton (B. Aires) 2020;89:217–227. doi: 10.32604/phyton.2020.08523. [DOI] [Google Scholar]
- 46.Van Loon LC. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 2007;119:243–254. doi: 10.1007/s10658-007-9165-1. [DOI] [Google Scholar]
- 47.Bakker PAHM, Pieterse CMJ, Van Loon LC. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology. 2007;97:239–243. doi: 10.1094/PHYTO-97-2-0239. [DOI] [PubMed] [Google Scholar]
- 48.Danish S, et al. Drought stress alleviation by ACC deaminase producing Achromobacter xylosoxidans and Enterobacter cloacae, with and without timber waste biochar in maize. Sustainability. 2020;12:6286. doi: 10.3390/su12156286. [DOI] [Google Scholar]
- 49.Liu W, Du L, Yang Q. Biogas slurry added amino acids decreased nitrate concentrations of lettuce in sand culture. Acta Agric. Scand. Sect. B Soil Plant Sci. 2009;59:260–264. [Google Scholar]
- 50.Figueiredo MVB, Martinez CR, Burity HA, Chanway CP. Plant growth-promoting rhizobacteria for improving nodulation and nitrogen fixation in the common bean (Phaseolus vulgaris L.) World J. Microbiol. Biotechnol. 2008;24:1187–1193. doi: 10.1007/s11274-007-9591-4. [DOI] [Google Scholar]
- 51.Shah AA, et al. Synergistic effect of bacillus thuringiensis iags 199 and putrescine on alleviating cadmium-induced phytotoxicity in capsicum annum. Plants. 2020;9:151. doi: 10.3390/plants9111512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbas M, et al. Gibberellic acid induced changes on growth, yield, superoxide dismutase, catalase and peroxidase in fruits of bitter gourd (Momordica charantia L.) Horticulturae. 2020;6:72. doi: 10.3390/horticulturae6040072. [DOI] [Google Scholar]
- 53.Adnan M, et al. Coupling phosphate-solubilizing bacteria with phosphorus supplements improve maize phosphorus acquisition and growth under lime induced salinity stress. Plants. 2020;9:900. doi: 10.3390/plants9070900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ullah A, et al. Impact of seed dressing and soil application of potassium humate on cotton plants productivity and fiber quality. Plants. 2020;9:1444. doi: 10.3390/plants9111444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zafar-ul-Hye M, et al. Potential role of compost mixed biochar with rhizobacteria in mitigating lead toxicity in spinach. Sci. Rep. 2020;10:69183. doi: 10.1038/s41598-020-69183-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaymak HC, Yarali F, Guvenc I, Figen Donmez M. The effect of inoculation with plant growth rhizobacteria (PGPR) on root formation of mint (Mentha piperita L.) cuttings. Afr. J. Biotechnol. 2008;7:4479–4483. [Google Scholar]
- 57.Lawrence A, et al. The UKIRT infrared deep sky survey (UKIDSS) Mon. Not. R. Astron. Soc. 2007;379:1599–1617. doi: 10.1111/j.1365-2966.2007.12040.x. [DOI] [Google Scholar]
- 58.EXCELLING THE NORTH AMERICAN MINT INDUSTRY. Mint Industry Research Councilhttps://www.usmintindustry.com/ (2010).
- 59.Zheljazkov VD, Cantrell CL, Astatkie T, WayneEbelhar M. Productivity, oil content, and composition of two spearmint species in Mississippi. Agron. J. 2010;102:129–133. doi: 10.2134/agronj2009.0258. [DOI] [Google Scholar]
- 60.Ward NI, Savage JM. Metal dispersion and transportational activities using food crops as biomonitors. Sci. Total Environ. 1994;146–147:309–319. doi: 10.1016/0048-9697(94)90251-8. [DOI] [PubMed] [Google Scholar]
- 61.Waseem A, et al. Pollution status of Pakistan: a retrospective review on heavy metal contamination of water, soil, and vegetables. Biomed Res. Int. 2014;2014:813206. doi: 10.1155/2014/813206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thies, J. & Rillig, M. C. Characteristics of biochar: Biological properties. In Biochar for Environmental Management: Science and Technology. (2009).
- 63.Novak JM, et al. Short-term CO2 mineralization after additions of biochar and switchgrass to a Typic Kandiudult. Geoderma. 2010;154:281–288. doi: 10.1016/j.geoderma.2009.10.014. [DOI] [Google Scholar]
- 64.Cheng CH, Lehmann J, Thies JE, Burton SD, Engelhard MH. Oxidation of black carbon by biotic and abiotic processes. Org. Geochem. 2006;37:1477–1488. doi: 10.1016/j.orggeochem.2006.06.022. [DOI] [Google Scholar]
- 65.Cheng CH, Lehmann J, Engelhard MH. Natural oxidation of black carbon in soils: changes in molecular form and surface charge along a climosequence. Geochim. Cosmochim. Acta. 2008;72:1598–1610. doi: 10.1016/j.gca.2008.01.010. [DOI] [Google Scholar]
- 66.Etesami, H. & Adl, S. M. Plant Growth-Promoting Rhizobacteria (PGPR) and Their Action Mechanisms in Availability of Nutrients to Plants. In Phyto-Microbiome in Stress Regulation (eds. Kumar, M., Kumar, V. & Prasad, R.) 147–203 (Springer, 2020). 10.1007/978-981-15-2576-6_9.
- 67.Nardi, S., Carletti, P., Pizzeghello, D. & Muscolo, A. Biological activities of humic substances, in biophysicochemical processes involving natural nonliving organic matter in environmental systems. In Fundamentals and Impact of Mineral-Organic-Biota Interactions on the Formation, Transformation, Turnover, and Storage of Natural Nonliving Organic Matter (NOM) (eds. Senesi, N. et al.) (Wiley, 2009).
- 68.Li M, et al. Population characteristics and influential factors of nitrogen cycling functional genes in heavy metal contaminated soil remediated by biochar and compost. Sci. Total Environ. 2019;651:2166–2174. doi: 10.1016/j.scitotenv.2018.10.152. [DOI] [PubMed] [Google Scholar]
- 69.Zhang J, et al. Ammonia-oxidizing bacterial communities and shaping factors with different: Phanerochaete chrysosporium inoculation regimes during agricultural waste composting. RSC Adv. 2016;6:61473–61481. doi: 10.1039/C6RA04817J. [DOI] [Google Scholar]
- 70.Laird D, Fleming P, Wang B, Horton R, Karlen D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma. 2010;158:436–442. doi: 10.1016/j.geoderma.2010.05.012. [DOI] [Google Scholar]
- 71.Brodowski S, Amelung W, Haumaier L, Abetz C, Zech W. Morphological and chemical properties of black carbon in physical soil fractions as revealed by scanning electron microscopy and energy-dispersive X-ray spectroscopy. Geoderma. 2005;128:116–129. doi: 10.1016/j.geoderma.2004.12.019. [DOI] [Google Scholar]
- 72.Egamberdieva D, Davranov K, Wirth S, Hashem A, AbdAllah EF. Impact of soil salinity on the plant-growth—promoting and biological control abilities of root associated bacteria. Saudi J. Biol. Sci. 2017;24:1601–1608. doi: 10.1016/j.sjbs.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Spokas KA, Koskinen WC, Baker JM, Reicosky DC. Impacts of woodchip biochar additions on greenhouse gas production and sorption/degradation of two herbicides in a Minnesota soil. Chemosphere. 2009;77:574–581. doi: 10.1016/j.chemosphere.2009.06.053. [DOI] [PubMed] [Google Scholar]
- 74.Cao X, Ma L, Gao B, Harris W. Dairy-manure derived biochar effectively sorbs lead and atrazine. Environ. Sci. Technol. 2009;43:3285–3291. doi: 10.1021/es803092k. [DOI] [PubMed] [Google Scholar]
- 75.Zhang A, et al. Effect of biochar amendment on yield and methane and nitrous oxide emissions from a rice paddy from Tai Lake plain, China. Agric. Ecosyst. Environ. 2010;139:469–475. doi: 10.1016/j.agee.2010.09.003. [DOI] [Google Scholar]
- 76.O’Neill B, et al. Bacterial community composition in Brazilian Anthrosols and adjacent soils characterized using culturing and molecular identification. Microb. Ecol. 2009;58:23–35. doi: 10.1007/s00248-009-9515-y. [DOI] [PubMed] [Google Scholar]
- 77.Joseph S, et al. Effects of enriched biochars containing magnetic iron nanoparticles on mycorrhizal colonisation, plant growth, nutrient uptake and soil quality improvement. Pedosphere. 2015;25:749–760. doi: 10.1016/S1002-0160(15)30056-4. [DOI] [Google Scholar]
- 78.Musadji NY, et al. Spectral characteristics of soil dissolved organic matter: long-term effects of exogenous organic matter on soil organic matter and spatial-temporal changes. Chemosphere. 2020;240:124808. doi: 10.1016/j.chemosphere.2019.124808. [DOI] [PubMed] [Google Scholar]
- 79.El-Naggar AH, et al. Carbon mineralization and nutrient availability in calcareous sandy soils amended with woody waste biochar. Chemosphere. 2015;138:67–73. doi: 10.1016/j.chemosphere.2015.05.052. [DOI] [PubMed] [Google Scholar]
- 80.Ippolito JA, Laird DA, Busscher WJ. Environmental benefits of biochar. J. Environ. Qual. 2012;41:967–972. doi: 10.2134/jeq2012.0151. [DOI] [PubMed] [Google Scholar]
- 81.Liang B, et al. Black carbon increases cation exchange capacity in soils. Soil Sci. Soc. Am. J. 2006;70:1719–1730. doi: 10.2136/sssaj2005.0383. [DOI] [Google Scholar]
- 82.Prakash, J., Ram, V. & Meena, S. Potassium-solubilizing bacteria and their application in agriculture. In Potassium Solubilizing Microorganisms for Sustainable Agriculture 293–313 (Springer, 2016). 10.1007/978-81-322-2776-2.
- 83.Ahmad M, Zahir ZA, Asghar HN, Arshad M. The combined application of rhizobial strains and plant growth promoting rhizobacteria improves growth and productivity of mung bean (Vigna radiata L.) under salt-stressed conditions. Ann. Microbiol. 2012;62:1321–1330. doi: 10.1007/s13213-011-0380-9. [DOI] [Google Scholar]
- 84.Yermiyahu U, et al. Nitrogen, phosphorus, and potassium uptake by wheat and their distribution in soil following successive, annual compost applications. J. Environ. Qual. 2004;33:1855–1865. doi: 10.2134/jeq2004.1855. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z, Dong X, Wang S, Pu X. Benefits of organic manure combined with biochar amendments to cotton root growth and yield under continuous cropping systems in Xinjiang, China. Sci. Rep. 2020;10:4718. doi: 10.1038/s41598-020-61118-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xie H, Pasternak JJ, Glick BR. Isolation and characterization of mutants of the plant growth-promoting rhizobacterium Pseudomonas putida GR12-2 that overproduce indoleacetic acid. Curr. Microbiol. 1996;32:67–71. doi: 10.1007/s002849900012. [DOI] [Google Scholar]
- 87.Mohite B. Isolation and characterization of indole acetic acid ( IAA) producing bacteria from rhizospheric soil and its effect on plant growth. J. Sci. Plant Nutr. 2013;13:638–649. [Google Scholar]
- 88.Gupta A, Meyer JM, Goel R. Development of heavy metal-resistant mutants of phosphate solubilizing Pseudomonas sp. NBRI 4014 and their characterization. Curr. Microbiol. 2002;45:323–327. doi: 10.1007/s00284-002-3762-1. [DOI] [PubMed] [Google Scholar]
- 89.Younis, U. et al. Agric. Environ. Sci. Pollut. Res.23, 21385–21394 (2016).
- 90.Burd GI, Dixon DG, Glick BR. A plant growth-promoting bacterium that decreases nickel toxicity in seedlings. Appl. Environ. Microbiol. 1998;64:3663–3668. doi: 10.1128/AEM.64.10.3663-3668.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Glick BR. The enhancement of plant growth by free-living bacteria. Can. J. Microbiol. 1995;41:109–117. doi: 10.1139/m95-015. [DOI] [Google Scholar]
- 92.Haritash AK, Kaushik CP. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J. Hazard. Mater. 2009;169:1–15. doi: 10.1016/j.jhazmat.2009.03.137. [DOI] [PubMed] [Google Scholar]
- 93.Song QJ, Greenway GM. A study of the elemental leachability and retention capability of compost. J. Environ. Monit. 2004;6:31–37. doi: 10.1039/b310840f. [DOI] [PubMed] [Google Scholar]
- 94.Yuan JH, Xu RK, Zhang H. The forms of alkalis in the biochar produced from crop residues at different temperatures. Bioresour. Technol. 2011;102:3488–3497. doi: 10.1016/j.biortech.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 95.Artelle LYHW, Odgers JAER. Immobilization of heavy metal ions (Cu II, Cd II, Ni II, and Pb II) by Broiler Litter-derived biochars in water and soil. J. Agric. Food Chem. 2010;58:5538–5544. doi: 10.1021/jf9044217. [DOI] [PubMed] [Google Scholar]
- 96.Ahmad M, et al. Biochar as a sorbent for contaminant management in soil and water: a review. Chemosphere. 2014;99:19–33. doi: 10.1016/j.chemosphere.2013.10.071. [DOI] [PubMed] [Google Scholar]
- 97.McGrath SP, Zhao FJ, Lombi E. Plant and rhizosphere processes involved in phytoremediation of metal-contaminated soils. Plant Soil. 2001;232:207–214. doi: 10.1023/A:1010358708525. [DOI] [Google Scholar]
- 98.Lasat MM. Phytoextraction of toxic metals: a review of biological mechanisms. J. Environ. Qual. 2002;31:109–120. [PubMed] [Google Scholar]
- 99.Dworkin M, Foster JW. Experiments with some microorganisms which utilize ethane and hydrogen. J. Bacteriol. 1958;75:592–603. doi: 10.1128/JB.75.5.592-603.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Awashthi, S. K. Prevention of Food Adultration. (Ashoka Law House, 2000).
- 101.Moodie CD, Smith HW, Creery RAM. Laboratory Manual for Soil Fertility. Dept. of Agron; 1959. [Google Scholar]
- 102.Walkley A, Black IA. An examination of the degtjareff method for determining soil organic matter, and a proposed modification of the chromic acid titration method. Soil Sci. 1934;37:29–38. doi: 10.1097/00010694-193401000-00003. [DOI] [Google Scholar]
- 103.Watanabe FS, Olsen SR. Test of an ascorbic acid method for determining phosphorus in water and NaHCO3 extracts from Soil1. Soil Sci. Soc. Am. J. 1965;29:677. doi: 10.2136/sssaj1965.03615995002900060025x. [DOI] [Google Scholar]
- 104.Nadeem F, et al. Qualitative and chemical analysis of rice kernel to time of application of phosphorus in combination with zinc under anaerobic conditions. Asian J. Agric. Biol. 2013;1:67–75. [Google Scholar]
- 105.Arnon DI. Copper enzymes in isolated chloroplasts. polyphenoloxidase in beta vulgaris. Plant Physiol. 1949;24:1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones, J. B., WolfH, B. & Mills, H. A. Plant Analysis Handbook: A Practical Sampling, Preparation, Analysis, and Interpretation Guide. (Micro-Macro Publishing Inc., 1991).
- 107.Chapman HD, Pratt PF. Methods of analysis for soils, plants and water. University of California; 1961. [Google Scholar]
- 108.Steel, R. G., Torrie, J. H. & Dickey, D. A. Principles and Procedures of Statistics: A Biometrical Approach. (McGraw Hill Book International Co., 1997).