Figure 2. Effect of Semaglutide, 2.4 mg Once Weekly, Compared With Placebo on Efficacy Outcomes During the Entire Trial (Full Analysis Set).
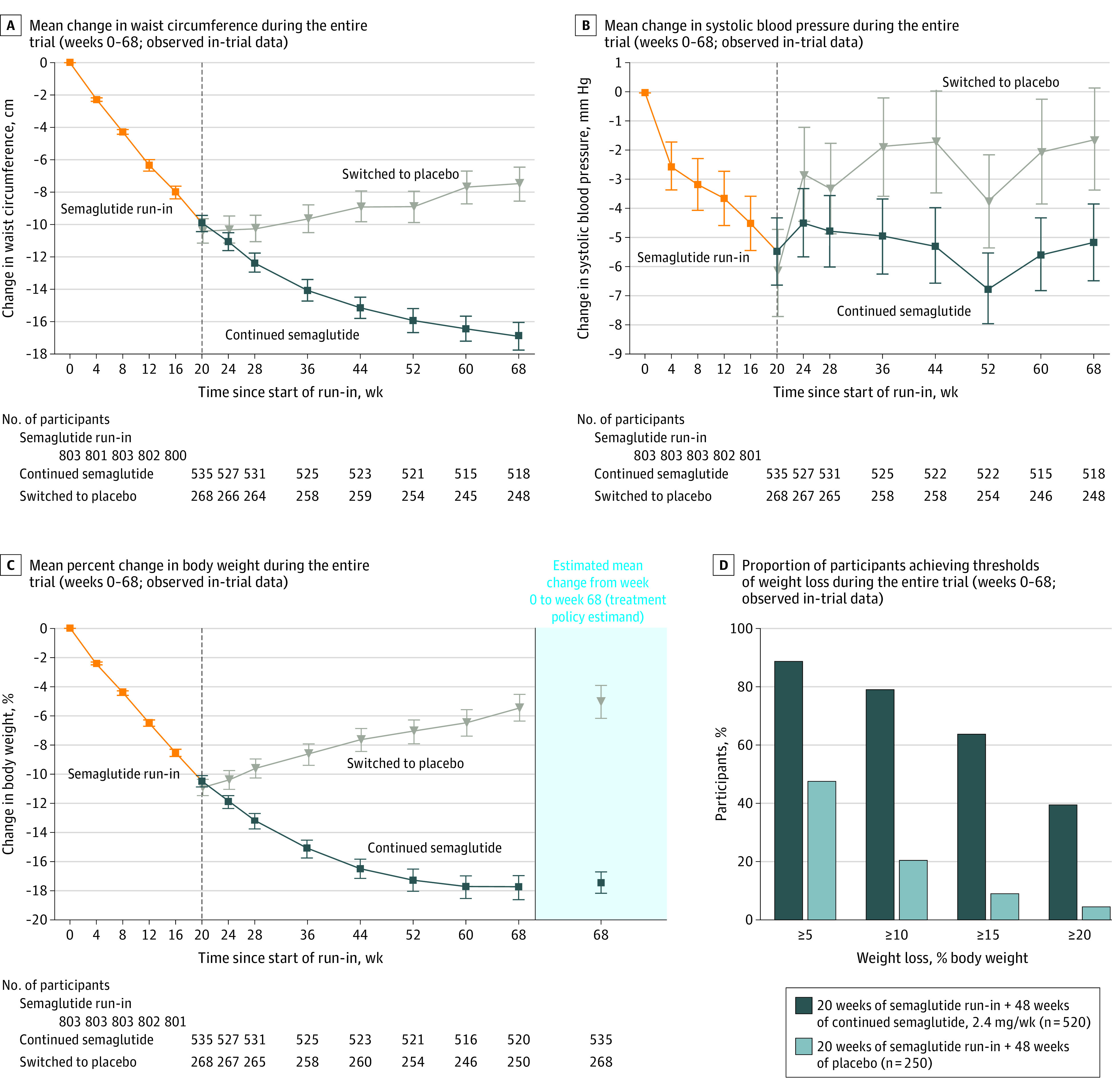
Data presented in panels A, B, and C are observed data for the full analysis set from the in-trial period (the time from week 0 to the date of last contact with trial site). Error bars represent 95% confidence intervals for the mean. Participant numbers shown denote those contributing to the mean. The dashed vertical line at week 20 represents the randomization time point. Data in the shaded area on the right in panel C are estimated mean changes from week 0 to week 68 for the treatment policy estimand, analyzed using the full analysis set. (The treatment policy estimand assessed the treatment effect regardless of treatment discontinuation or rescue intervention using analysis of covariance, with randomized treatment as a factor and baseline end point value as a covariate, and a multiple imputation approach for missing data.10) Data in panel D are observed data among all randomized participants with a week 68 assessment from the in-trial period (the time from week 0 to the date of last contact with trial site).
