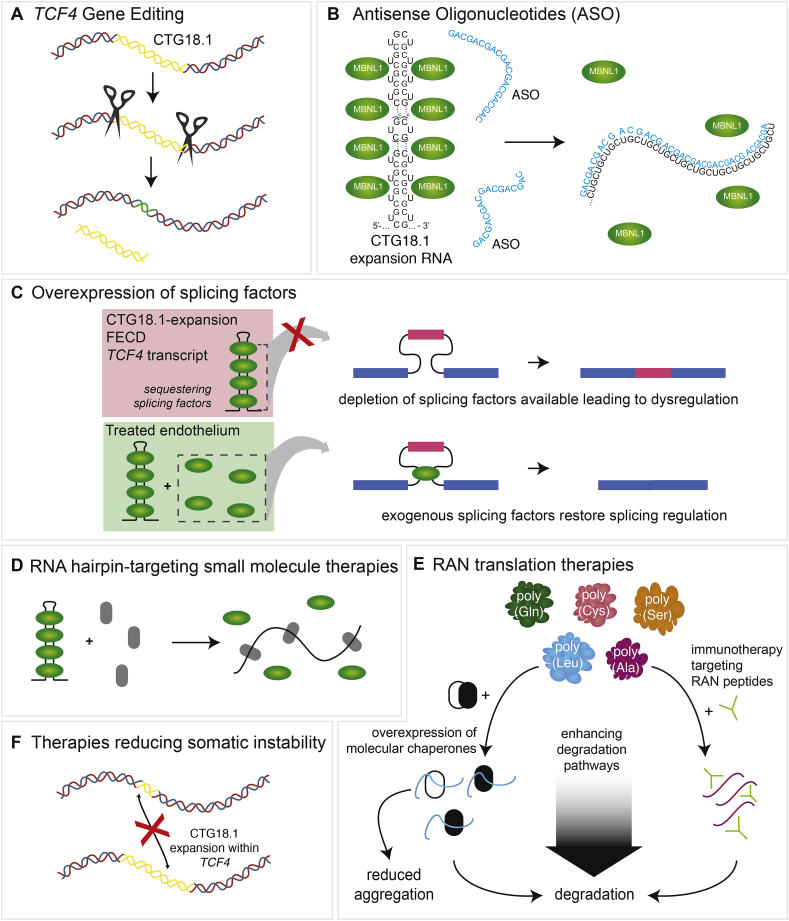
Fig. 9.
Potential therapeutic strategies targeting pathogenic mechanisms associated with CTG18.1 expansion-mediated Fuchs endothelial corneal dystrophy (FECD). A. Gene editing tools may be applied in the future to reduce the size or presence of CTG18.1 expansions. The CTG18.1 repeat element is represented as yellow. B. Antisense oligonucleotide (ASO) therapeutic strategies can target (CUG)n or (CAG)n RNA transcripts derived from expanded copies of CTG18.1 to physically block the formation of foci and or induce degradation of such transcripts. C. Overexpression of splicing factors, such as MBNL proteins, could restore splicing regulation. D. Small molecule therapeutics (grey) could induce disruption of RNA hairpins on (CUG)n or (CAG)n RNA transcripts, releasing sequestered splicing factors (green). E. Various strategies targeting RAN translation may in the future prove to have therapeutic benefit. Immunotherapy targeting RAN peptides would enable the cell to degrade the peptide aggregates. Overexpression of molecular chaperones could potentially prevent the aggregation of RAN peptides and/or enhance degradation levels. F. Therapeutics aimed at reducing levels of somatic and age-related levels of repeat instability may in the future prove to have therapeutic benefit for CTG18.1-expansion associated FECD.