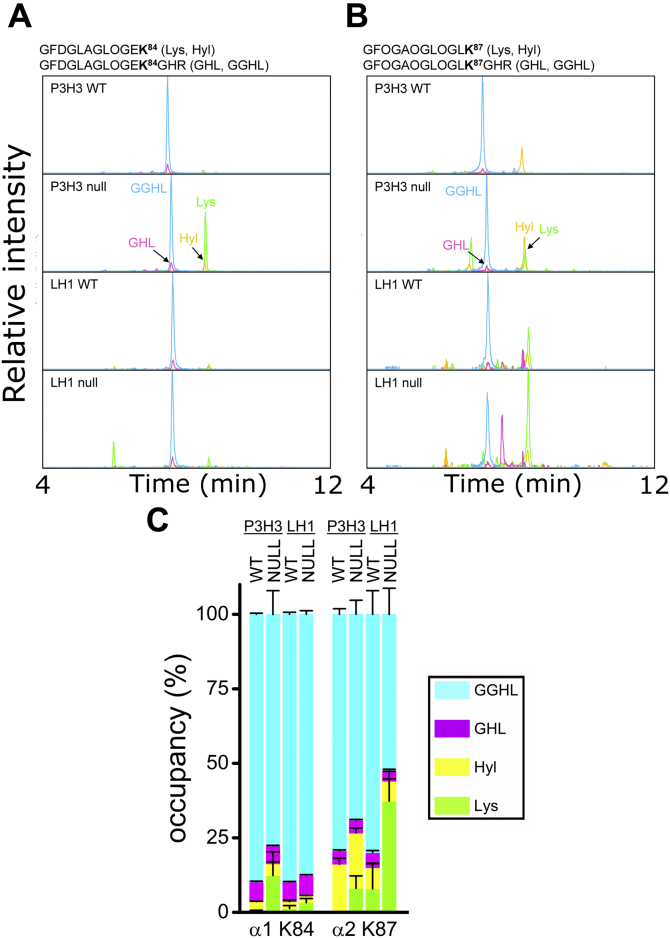
Figure 3.
Characterization of lysine posttranslational modifications at individual sites of skin type V collagen from P3H3 and LH1 null mice.A, representative extracted ion chromatograms of α1(V) K84-containing tryptic peptides (m/z 588.7911 ± 0.02 (z = 2) for Lys, m/z 596.7886 ± 0.02 (z = 2) for Hyl, m/z 568.9372 ± 0.02 (z = 3) for GHL, and m/z 622.9548 ± 0.02 (z = 3) for GGHL). B, representative extracted ion chromatograms of α2(V) K87-containing tryptic peptides (m/z 579.8040 ± 0.02 (z = 2) for Lys, m/z 587.8015 ± 0.02 (z = 2) for Hyl, m/z 562.9458 ± 0.02 (z = 3) for GHL, and m/z 616.9634 ± 0.02 (z = 3) for GGHL). C, bar graphs represent the occupancy of lysine modifications in individual lysyl hydroxylation sites of skin type V collagen of P3H3 null and LH1 null mice and their WT controls. Individual values [GGHL (cyan); glucosylgalactosyl hydroxylysine, GHL (magenta); galactosyl hydroxylysine, Hyl (yellow); unmodified hydroxylysine, Lys (green); unmodified lysine] correspond to Table 2. Values of amino acids were obtained using mass spectrometry, and biological replicates were as shown in Table 2. α1, α2, and K + numbers indicate the α1 and α2 chain of type V collagen and residue number from the first residue of the triple helical domain, respectively.