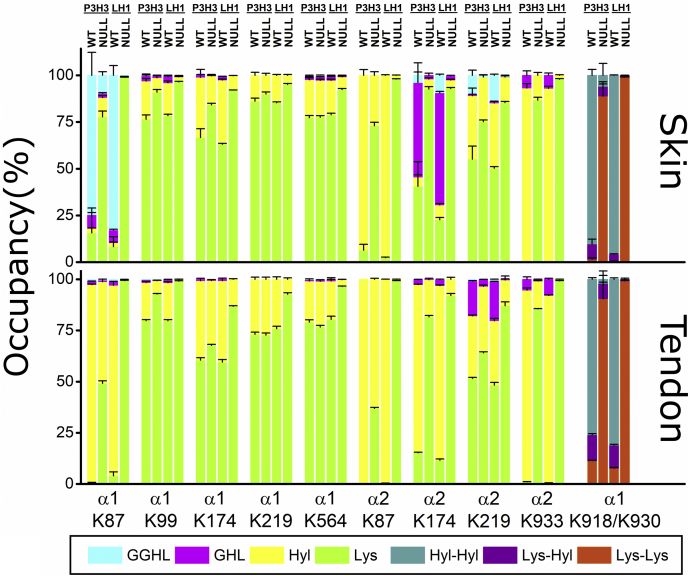
Figure 6.
Characterization of lysine posttranslational modifications at individual sites of skin and tendon type I collagen from P3H3 and LH1 null mice.Bar graphs represent the occupancy of lysine modifications in individual lysyl hydroxylation sites of the skin and tendon type I collagen of P3H3 null and LH1 null mice and their WT controls. The lysine modification sites α1 K918 and K930 were included in the same peptide GDKGETGEQGDRGIKGER. Individual values [GGHL (cyan); glucosylgalactosyl hydroxylysine, GHL (magenta); galactosyl hydroxylysine, Hyl (yellow); unmodified hydroxylysine, Lys (green); unmodified lysine, Hyl-Hyl (dark cyan); unmodified hydroxylysine and unmodified hydroxylysine, Lys-Hyl (purple); unmodified lysine and unmodified hydroxylysine, Lys-Lys (orange); unmodified lysine and unmodified lysine] correspond to Tables 4 and 5 for the skin and tendon, respectively. Values of modified and unmodified hydroxylysine and unmodified lysine were obtained using mass spectrometry, and the number of biological replicates was n = 4 for all tissues and genotypes. α1, α2, and K + numbers indicate the α1 and α2 chain of type I collagen and residue number from the first residue of the triple helical domain, respectively.