Abstract
Background:
At least 40% of individuals with multiple sclerosis (MS) exercise chronic insomnia, and the prevalence is likely higher due to underdiagnosis. Poor sleep quality has been associated with increased fatigue, anxiety, depression, and risk of relapse in individuals with MS. While cognitive behavioral therapy for insomnia (CBT-I) is the recommended treatment for chronic insomnia, the treatment effect of CBT-I in people with MS is unclear.
Objective:
This pilot randomized control trial (RCT) assessed the feasibility and treatment effect of CBT-I to improve sleep quality and fatigue in individuals with MS with symptoms of insomnia.
Methods:
Thirty-three individuals with MS (30 females, 3 males; 30 relapsing-remitting; 3 secondary-progressive; 53.0 ± 9.4 years old) with symptoms of insomnia were randomized into one of three arms: 1. 6-week CBT-I program, 2. 6-week active control, or 3. Single session of sleep education. Participants completed surveys to assess sleep quality, fatigue, sleep self-efficacy, depression, and anxiety.
Results:
CBT-I in individuals with MS is feasible with high retention and adherence rate. All groups experienced a large magnitude of improvement in insomnia symptoms. The CBT-I and brief education groups experienced a large magnitude of improvement in sleep quality and fatigue. Only the CBT-I group demonstrated a large magnitude of improvement in sleep self-efficacy and depression.
Conclusion:
This is the first study to prospectively demonstrates that CBT-I is feasible in people with MS and produces promising improvements in insomnia severity, sleep quality, sleep self-efficacy and comorbid symptoms of fatigue, depression, and anxiety. Future studies are needed to determine mechanisms for these improvements and expand the scope of individuals with MS who may benefit from CBT-I. Furthermore, considering the moderate to large improvements experienced by the brief education group and the limited number of CBT-I providers, a stepped-care approach warrants consideration.
Trial Registry:
Clinicaltrials.gov, NCT03216889
https://clinicaltrials.gov/ct2/show/NCT03216889?term=sleep&recrs=e&cond=multiple+sclerosis&rank=9
Keywords: cognitive behavior therapy, insomnia, fatigue, sleep quality, multiple sclerosis
Introduction
Nearly 70% of individuals with multiple sclerosis (MS) report sleep disturbances,1 although the incidence is likely higher due to underreporting.2 Moreover, 40% or more of individuals with MS experience chronic insomnia,3 defined as difficulty falling asleep, maintaining sleep, or waking up too early at least 3 nights/week for the past 3 months.4 Sleep disturbances in individuals with MS can be caused by demyelination or degeneration of areas of the brain that control sleep5 or by physical and psychological factors such as pain, spasticity, medication, anxiety, depression, and bladder problems.6
Fatigue is the most common symptom, reported in up to 90% of individuals with MS.7 In 40% of individuals with MS, fatigue is reported as the worst symptom.8 Fatigue contributes to reduced quality of life, unemployment, and decreased participation in activities of daily living in individuals with MS.9,10 Sleep disturbances have also been associated with an increase in perceived fatigue in individuals with MS.11 Research suggests an increase in pro-inflammatory cytokines in the central nervous system of individuals with MS who have fatigue may contribute to an increased incidence of sleep disturbances.12 Furthermore, treatment of sleep disorders (e.g., insomnia, sleep apnea, and restless leg syndrome) reduces fatigue in individuals with MS.13
CBT-I is an effective multicomponent treatment strategy for insomnia which addresses behaviors and thoughts that can negatively impact sleep, and is the most prevalent recommended non-pharmacological treatment for chronic insomnia.14 CBT-I typically includes stimulus control, time in bed restriction, sleep hygiene education, cognitive strategies, and relaxation techniques.15 Research has demonstrated CBT-I results in improved sleep efficacy and sleep latency, reduced number of awakenings after sleep onset, and increased total sleep time in people with primary insomnia.16,15 Furthermore, CBT-I has been shown to be more effective long-term for treating primary insomnia or insomnia due to comorbid conditions compared to pharmacological interventions.17 Importantly, improvements in sleep outcomes remain at up to 10 years following CBT-I.18 In addition, recent meta-analyses14,19 determined CBT-I produced medium to large effect sizes on sleep outcomes in people with a variety of comorbid conditions. However, MS was not one of the medical conditions considered, and individuals with MS do not appear to have participated in any of the studies included in the meta-analyses.14,19
Emerging research indicates CBT-I is efficacious in treating insomnia symptoms in individuals with MS. A retrospective study of 11 individuals with MS who underwent CBT-I reported 86% of the participants experienced a reduction in insomnia symptoms, 50% experienced a reduction in depression symptoms, 60% reported a reduction in fatigue, and 73% reported an increase in total sleep time (M = 1.5 hours).20 However, the interpretation of these results is difficult given the study design, small sample size, and failure to account for comorbid sleep disorders. A recent case study of an individual with MS reported an improvement in sleep quality, life satisfaction, anxiety, and depression, and was able to reduce medication usage to none.21 While these studies demonstrate proof of concept, assessing the feasibility and treatment effect of CBT-I prospectively in individuals with MS with symptoms of insomnia is needed. Thus, the purpose of this pilot RCT was to assess the feasibility (primary aim) and treatment effect (secondary aim) of using CBT-I to improve MS symptoms of reduced sleep quality and fatigue in individuals with MS with symptoms of insomnia.
Materials and Methods
Participants were recruited from the MS specialty clinic at the University of Kansas Medical Center (KUMC), through newsletters and emails distributed by the National Multiple Sclerosis Society (NMSS), and from the KUMC Frontiers Research Participant Registry. Written informed consent was obtained from all study participants prior to enrollment. This study was approved by KUMC’s Institutional Review Board and was registered with clinicaltrials.gov ( NCT03216889).
To be included in the study, participants had to be: (1) 18–64 years old, (2) have relapsing-remitting or secondary progressive MS, (3) report difficulty falling asleep, maintaining sleep, or waking up too early at least 3 nights/week for the past 6 months, (4) score ≥10 on Insomnia Severity Index,22 (5) English speaking, and (6) score ≥ 24 on the Mini Mental State Exam (MMSE). Exclusion criteria included: (1) known untreated sleep disorder (i.e., sleep apnea or restless leg syndrome), (2) score >4 on STOP BANG (indicating elevated risk of sleep apnea), (3) increased risk of restless leg syndrome on RLS-Diagnosis Index, (4) score of ≥15 on the Patient Health Questionnaire (PHQ-9) indicating severe depression or endorse any suicidal ideation (answer 1, 2 or 3 on #9 of the PHQ-9),23 (6) history of alcohol/drug dependence or nervous system disorder other than MS, (7) severe neurological or sensory impairments that would interfere significantly with testing, (8) relapse and/or corticosteroid use in past 8 weeks, (9) performs shift work.
The feasibility of CBT-I was assessed using the following metrics: (1) number of people enrolled out of the number of people contacted (recruitment), (2) number of participants who completed the study (retention), (3) number of individuals who dropped out of the study (attrition), and (4) number of CBT-I sessions attended (adherence). To assess the treatment effect on sleep quality and fatigue, the Insomnia Severity Index (ISI),22 Pittsburgh Sleep Quality Index (PSQI),24 Fatigue Severity Scale (FSS),25 and Modified Fatigue Impact Scale (MFIS)26 were conducted at the baseline assessment and post-intervention. Research personnel conducting baseline and post-intervention reassessments were blinded to treatment group allocation.
The ISI22 assesses insomnia severity and consists of 7 questions each rated on a 0–4 scale. The range of scores on the ISI is 0–28, with ≥10 suggesting clinical insomnia.22 The PSQI assesses sleep quality and consists of 9 items within 7 sleep categories. The 7 sleep category scores are summed to form a single global score ranging from 0–21. A global score of >5 reflects poor sleep quality.24 The FSS assesses the impact of fatigue on activities for the week prior and consists of 9 questions.25 The mean of the 9 scores is calculated with a range of 0–7 with a higher score indicating greater fatigue impact. The MFIS assesses the impact of fatigue on daily activities for the month prior.26 The MFIS consists of 21 items with 3 subscales: physical, cognitive, and psychosocial. The score on the 21 items are scored with a range of 0–84 with a higher score indicating a greater impact of fatigue.
Because of their known bidirectional relationship with sleep and fatigue, depression (Patient Health Questionnaire, PHQ-9),23 anxiety (Generalized Anxiety Disorder Assessment, GAD-7),27 and sleep self-efficacy (SSE)28 were also gathered pre- and post-intervention. Participants provided a list of their medications at the baseline assessment. Participants were instructed to take their medication as prescribed by their physician during the study. Participants were asked to provide any changes to their medication at the reassessment.
Following baseline assessment, participants were randomized into one of three groups: CBT-I, active control (AC), or one-time brief education control (EC) group. The CBT-I program consisted of a 6-week, 1x/week for 45–60 minutes, in-person, one-on-one program with CS who is trained in providing CBT-I. A standardized CBT-I program was used based on the manual by Perlis et al.29 Participants maintained a sleep diary during the program to aid in tailoring the program and to determine time in bed prescription for the following week. Each session consisted of a review of material from the week(s) prior as needed, review of the participant’s weekly sleep log, calculation of sleep efficiency from the sleep log to determine if change in time in bed prescription was indicated, and discussion of challenges and successes. Video, demonstration, practice, and instructional handouts were used to supplement instruction. The standard weekly CBT-I sessions consisted of the following:
Week 1: education on the Behavioral Model of Insomnia30, discussion of rationale for time in bed restriction and stimulus control, discussion of sleep schedule, education for use of sleep diary, discussion of possible strategies to stay awake to prescribed bedtime and what to do if wake up in middle of night, and discussion and tailoring of general sleep hygiene recommendations. Modification of the recommendation to avoid naps was made for some individuals if they felt a nap was necessary for their health and wellbeing. Those individuals were recommended to limit napping to 30 minutes if possible, to take the nap as early in the day as possible, and to delay time in bed to fall asleep at night until adequately tired. Individuals with functional limitations or mobility difficulties or those concerned about falling were instructed to not get out of bed to perform stimulus control, but instead perform relaxation or distraction techniques in the bed.
Week 2: review of sleep hygiene recommendations, introduce diaphragmatic breathing and deep breathing technique.
Week 3: introduce mindfulness.
Week 4: introduce progressive muscle relaxation. Modifications were made for participants who had concern the contraction portion would aggravate their spasticity. Those individuals were instructed to focus on the relaxation component and not perform the contraction component.
Week 5: discussion of negative sleep beliefs if needed, reinforce positive changes and gains.
Week 6: discussion and assess global treatment gains, discuss relapse prevention.
Participants randomized into the AC group participated in gentle stretching and self-selected light or sedentary activity (i.e., playing games on the Wii, Uno, Sudoku, coloring in coloring book) with research personnel 1x/week for 6 weeks. Participants in the AC group started each session performing six stretches for the upper and lower extremities lead by a research assistant and guided by pictures with instructions. Stretching took approximately 5–10 minutes depending on the individual. Participants then selected which light or sedentary activity they would like to perform. Participants were instructed that they could perform the selected activity for the entire session or they could select another activity at any point during the session if they wanted. Each session lasted 45–60 minutes to match for amount of contact with research personnel as participants in the CBT-I group. Participants randomized into the EC group were provided a single-page handout of sleep promotion, and the education provided was tailored to each participant by a research assistant trained in providing CBT-I.
All data was analyzed using IBM SPSS Statistics version 22. One-ways ANOVAs and chi-square analyses were utilized to explore differences in demographics between the three groups at baseline. Feasibility of process was assessed using frequency analysis. To assess within group treatment effects on sleep, fatigue, depression, anxiety, and sleep self-efficacy, a repeated measures ANOVA model was used to generate parameter estimates to determine the significance of change from baseline to reassessment for each outcome of interest. Effect size (ES; Cohen’s d) including correlations between baseline and reassessment were also used to examine the magnitude of change in the outcome measures from baseline to reassessment. Cohen’s d was interpreted as small d= .2, medium d= .5, and large d= .8.31 To assess for difference in magnitude of change in outcomes from baseline to reassessment, one-way ANOVAs or ANCOVAs were used to assess for between group difference for change scores (reassessment score – baseline score) for each outcome of interest. The outcome at baseline was included in the ANCOVA as a covariate if there was a statistically significant between-group difference at baseline (ISI, PSQI, and MFIS). Alpha level was set at 0.05.
Results
The demographic information for each group is provided in Table 1. There were no significant differences between the groups for sex, race, ethnicity, disease type, age, disease duration, disease severity, or average number of medications (Table 1). There was no group difference in baseline performance on FSS (p= 0.170), PHQ-9 (p= 0.277), GAD-7 (p= 0.811), or SES (p= 0.128), but there were significant group differences on the ISI (p= 0.024), PSQI (p= 0.012), and MFIS (p= 0.022). Two individuals reported a change in medication at the reassessment; both individuals were in the brief education group, and both reported stopping a medication for depression.
Table 1.
Descriptive statistics of the participants. Data reported as mean (standard deviation).
| Group | CBT-I | AC | BE | p-value |
|---|---|---|---|---|
| Sex | 9 Females | 8 Females | 10 Females | 0.33 |
| 1 Male | 2 Males | 0 Males | ||
| Race | 9 White | 9 White | 10 White | 0.58 |
| 0 Black | 0 Black | 0 Black | ||
| 1 Unknown | 1 Unknown | |||
| Ethnicity | 9 Non-Hispanic | 9 Non-Hispanic | 10 No’-Hispanic | 0.58 |
| 0 Hispanic | 0 Hispanic | 0 Hispanic | ||
| 1 Unknown | 1 Unknown | |||
| MS Type | 8 RR | 10 RR | 9 RR | 0.33 |
| 2 SP | 0 SP | 1 SP | ||
| Age (years) | 51.1 (7.9) | 50.4 (12.4) | 56.9 (10.1) | 0.32 |
| PDDS | 1.3 (2.21) | 1.7 (2.3) | 2.0 (2.1) | 0.77 |
| Disease Duration (Years) | 17.3 (8.5) | 9.1 (8.9) | 18.3 (11.4) | 0.09 |
| Medications | 7.0 (4.8) | 5.0 (3.8) | 8.2 (5.5) | 0.244 |
| Range 0–16 | Range 0–13 | Range 0–19 | ||
| Classification (n)* | ||||
| Disease-modifying | 5 | 6 | 7 | |
| Spasticity | 4 | 3 | 5 | |
| Pain | 4 | 1 | 2 | |
| Depression or Anxiety | 4 | 2 | 3 | |
| Vitamin | 4 | 1 | 8 | |
| Stimulant | 0 | 1 | 0 | |
| Bladder activity | 2 | 1 | 2 | |
| Sleep aid | 2 | 0 | 2 | |
| Cardiac related | 5 | 4 | 4 | |
| Asthma | 1 | 1 | 0 | |
| Diabetes | 1 | 0 | 0 | |
| Allergy | 4 | 3 | 2 | |
| Other | 6 | 9 | 6 | |
| None | 1 | 1 | 0 |
RR: Relapsing Remitting, SP: Secondary Progressive, PDDS: Patient Determined Disease Steps.
Number of individuals taking a medication within the listed classification. Individuals may be taking more than one medication within the classification.
Feasibility
Of the 561 people contacted, 33 people were enrolled in the study for a recruitment rate of 5.9% (Figure 1). If only the 375 people that research personnel were able to contact are considered, the recruitment rate is 8.8%. The overall retention rate was 90.9% (30 out of 33 people total). The retention rate for each group was as follows: 10 out of 12 or 83.3% in the CBT-I group; 10 out of 10 or 100% for the AC group; and 10 out of 11 or 90.9% in the BE group. The overall attrition rate was 9.1% (3 out of 33). The attrition rate for each group was as follows: 2 out of 12 or 16.7% in the CBT-I group; 0 out of 10 for the AC group; and 1 out of 11 or 9.1% in the BE group. Adherence rate or the number of sessions attended on average by the CBT-I group was 5.6 sessions out of 6 or 93.3%, and 4.9 sessions out of 6 or 81.7% for the AC group.
Figure 1.
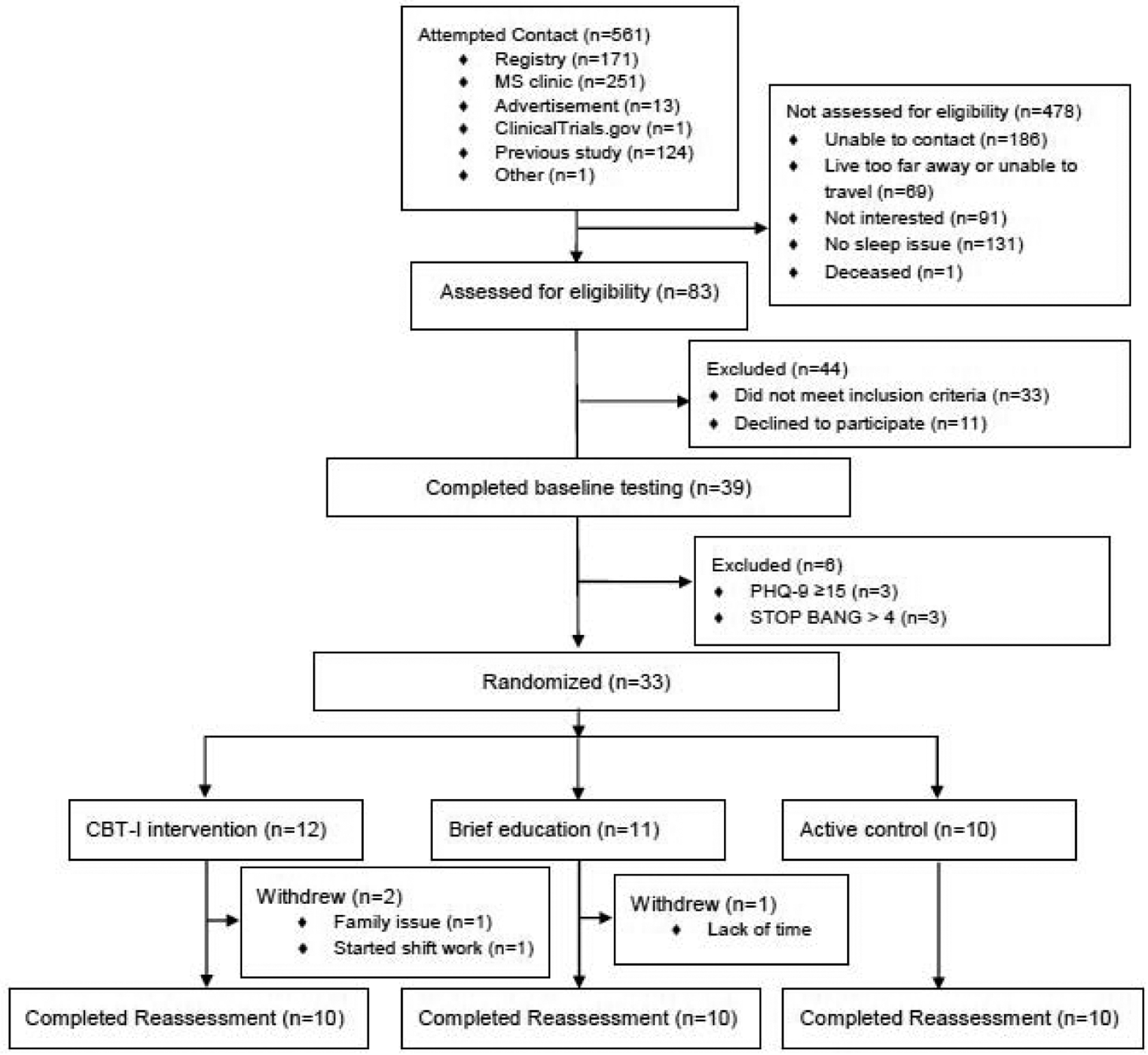
CONSORT flow diagram
Treatment Effect: Primary outcome measures
All groups showed a significant large reduction from baseline to reassessment on the ISI with the CBT-I group demonstrating the largest change (Table 2; Figure 2).
Table 2.
Primary and secondary outcomes at baseline (pre), reassessment (post), change score, and effect size. Data reported as mean (standard deviation). ISI: Insomnia Severity Index; PSQI: Pittsburgh Sleep Quality Index; MFIS: Modified Fatigue Impact Scale; FSS: Fatigue Severity Scale; SSE: Sleep Self-efficacy; PHQ-9: Patient Health Questionnaire, 9 items; GAD-7: Generalized Anxiety Disorder Assessment, 7 items; ES = Effect Size
| CBT-I | Active Control | Brief Education | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | Post | Change | p | ES | Pre | Post | Change | p | ES | Pre | Post | Change | p | ES | |
| Primary Outcomes | |||||||||||||||
| ISI | 18.1 (4.0) | 4.8 (2.5) | −13.8 (5.8) | <.001 | 2.729 | 13.7 (3.7) | 7.9 (3.5) | −5.8 (5.7) | .002 | 1.018 | 17.1 (2.7) | 10.0 (2.9) | −8.1 (4.4) | <.001 | 2.570 |
| PSQI | 13.1 (3.4) | 6.4 (2.6) | −6.7 (2.9) | <.001 | 2.314 | 9.4 (2.9) | 8.5 (2.6) | −0.9 | .269 | 0.506 | 12.5 (1.7) | 7.9 (1.8) | −4.6 (2.6) | <.001 | 1.672 |
| MFIS | 34.1 (18.2) | 14.8 (8.8) | −19.3 (18.2) | <.001 | 1.064 | 31.2 (17.9) | 29.5 (16.8) | −1.7 (8.9) | .716 | 0.192 | 50.7 (10.1) | 32.6 (14.1) | −18.1 (15.2) | 0.001 | 1.191 |
| FSS | 33.9 (14.7) | 25.4 (7.6) | −8.5 (16.2) | .043 | 0.524 | 34.4 (16.5) | 31.0 (17.3) | −3.4 (10.4) | .404 | 0.325 | 44.7 (10.4) | 41.6 (12.7) | −3.1 (10.5) | .446 | 0.292 |
| Secondary Outcomes | |||||||||||||||
| SSE | 24.3 (5.3) | 35.5 (5.7) | 11.2 (9.2) | <.001 | 1.221 | 29.0 (3.7) | 30.1 (4.2) | 1.1 (5.5) | .639 | 0.197 | 25.5 (6.2) | 30.4 (6.8) | 4.9 (6.6) | .044 | 0.740 |
| PHQ-9 | 7.5 (3.6) | 2.8 (4.3) | −7.0 (6.5) | <.001 | 0.798 | 5.8 (4.2) | 5.4 (4.0) | −0.4 | .777 | 0.212 | 8.2 (1.8) | 5.9 (3.3) | −4.7 (3.5) | .002 | 0.559 |
| GAD-7 | 5.2 (3.3) | 3.3 (4.2) | −7.0 (6.5) | .049 | 0.549 | 4.2 (4.0) | 4.2 (2.7) | 0.0 (2.4) | 1.00 | 0.000 | 4.8 (3.0) | 4.3 (4.0) | −0.5 | .592 | 0.181 |
Figure 2.
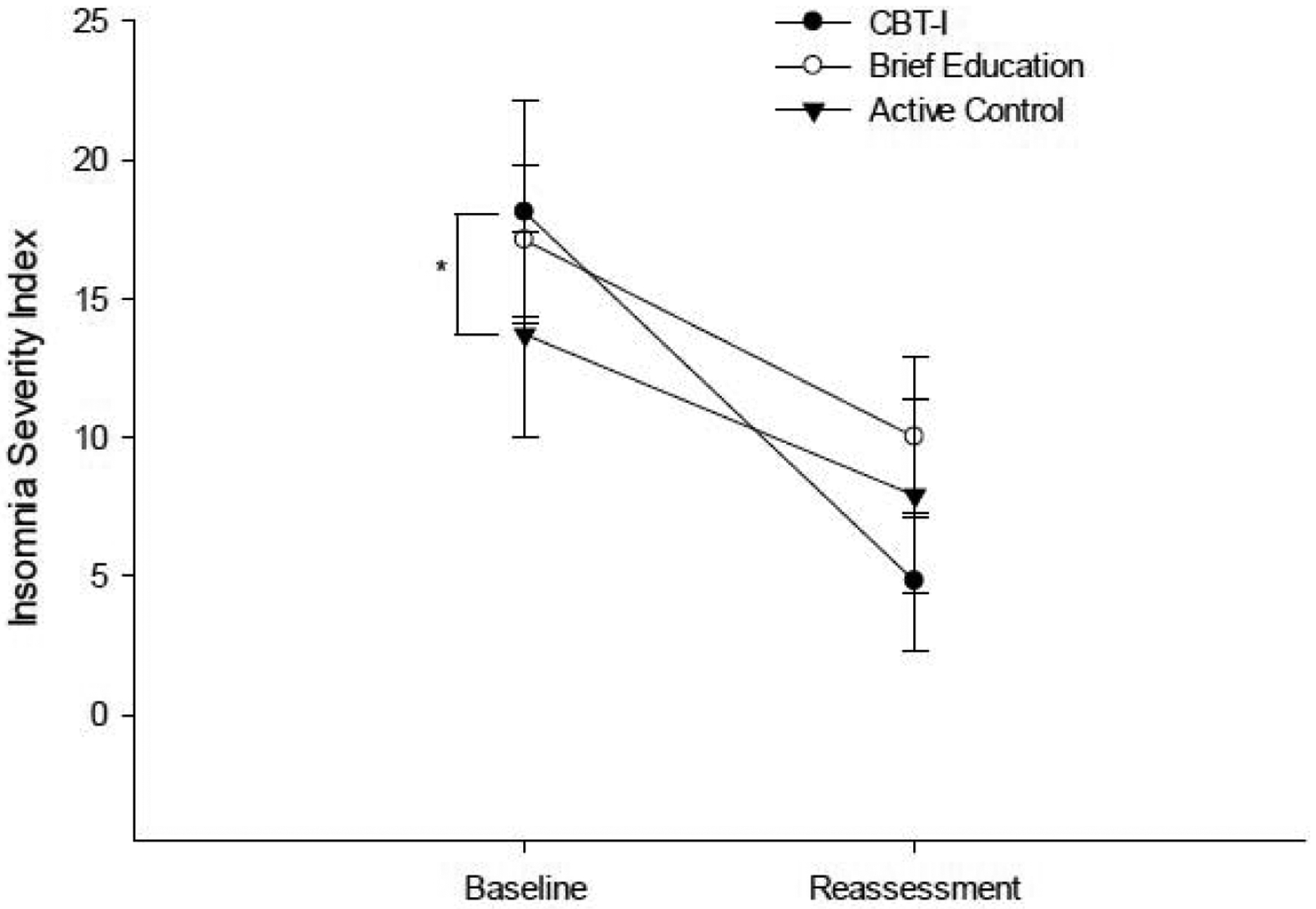
Performance on the ISI at baseline and post-intervention reassessment. *indicates significant group difference at baseline.
There was a significant difference in magnitude of change on the ISI between the CBT-I group and the BE group (p= 0.026; Table 3), but not between the CBT-I group and the AC group (p= 0.340).
Table 3.
p-values for between group comparisons on magnitude of change for primary and secondary outcomes. ISI: Insomnia Severity Index; PSQI: Pittsburgh Sleep Quality Index; MFIS: Modified Fatigue Impact Scale; FSS: Fatigue Severity Scale; SSE: Sleep Self-efficacy; PHQ-9: Patient Health Questionnaire, 9 items; GAD-7: Generalized Anxiety Disorder Assessment, 7 items;
| Change in Primary Outcomes | ||||
|---|---|---|---|---|
| ISI | PSQI | MFIS | FSS | |
| CBT-I vs AC | .340 | .005 | .018 | 1.00 |
| CBT-I vs BE | .026 | .202 | .258 | 1.00 |
| AC vs BE | 1.00 | .182 | 1.00 | 1.00 |
| Secondary Outcomes | ||||
| SSE | PHQ-9 | GAD-7 | ||
| CBT-I vs AC | .014 | .007 | .469 | |
| CBT-I vs BE | .195 | .763 | .877 | |
| AC vs BE | .768 | .115 | 1.00 | |
The CBT-I and BE groups showed a significant large improvement on the PSQI and MFIS (Table 2; Figures 3 and 4), with the CBT-I group demonstrating the largest magnitude of change on the PSQI (ES = 2.314) and similar large magnitude of change on the MFIS (ES = 1.064) as the BE group (ES = 1.191). There was a significant difference in magnitude of change on the PSQI and MFIS between the CBT-I group and the AC group (Table 3; PSQI p= 0.005; MFIS p= 0.018), but not between the CBT-I group and the BE group (Table 3; PSQI p= 0.202; MFIS p= 0.258; Figures 3 & 4).
Figure 3.
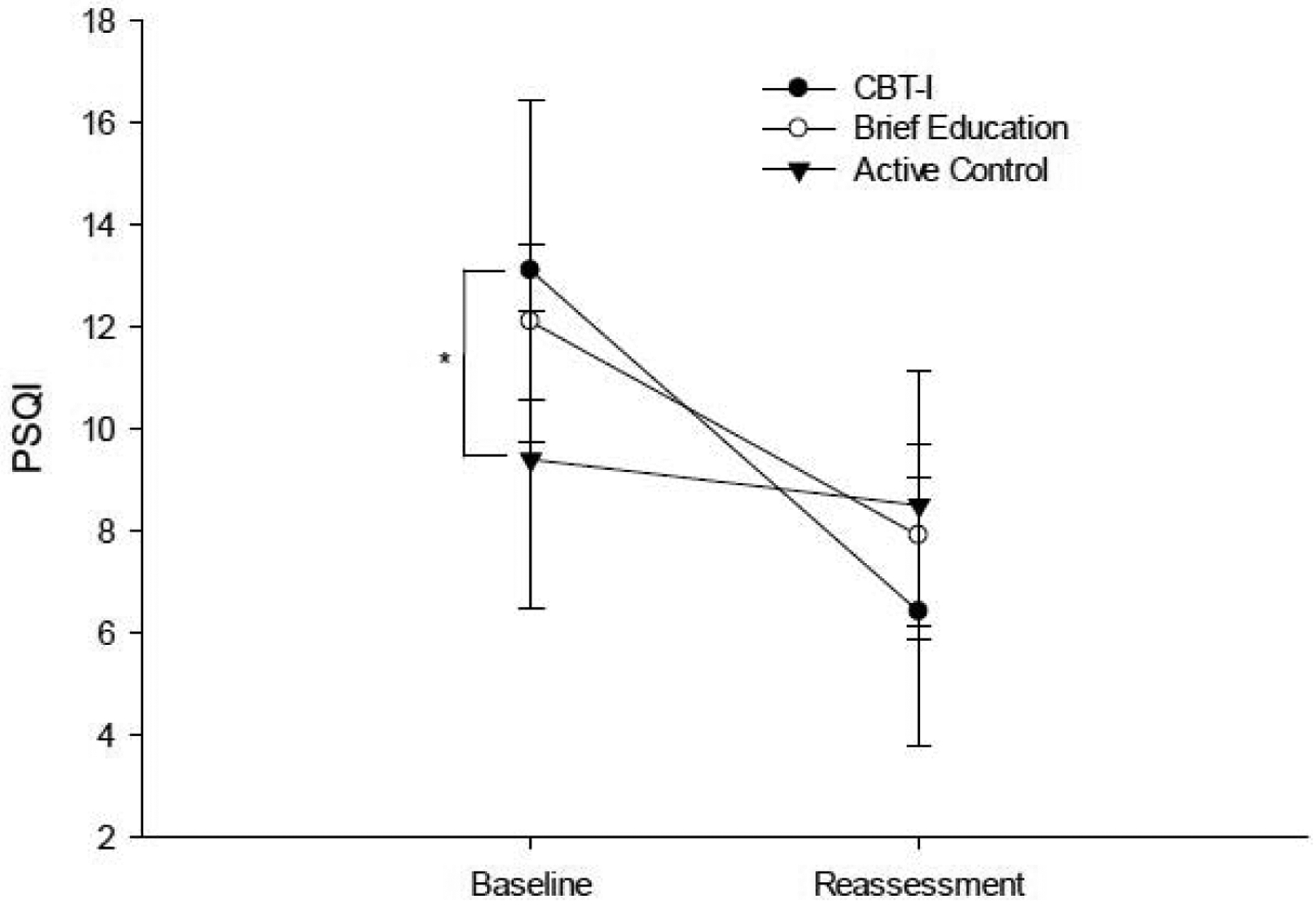
Performance on PSQI at baseline and post-intervention reassessment. *indicates significant group difference at baseline.
Figure 4.
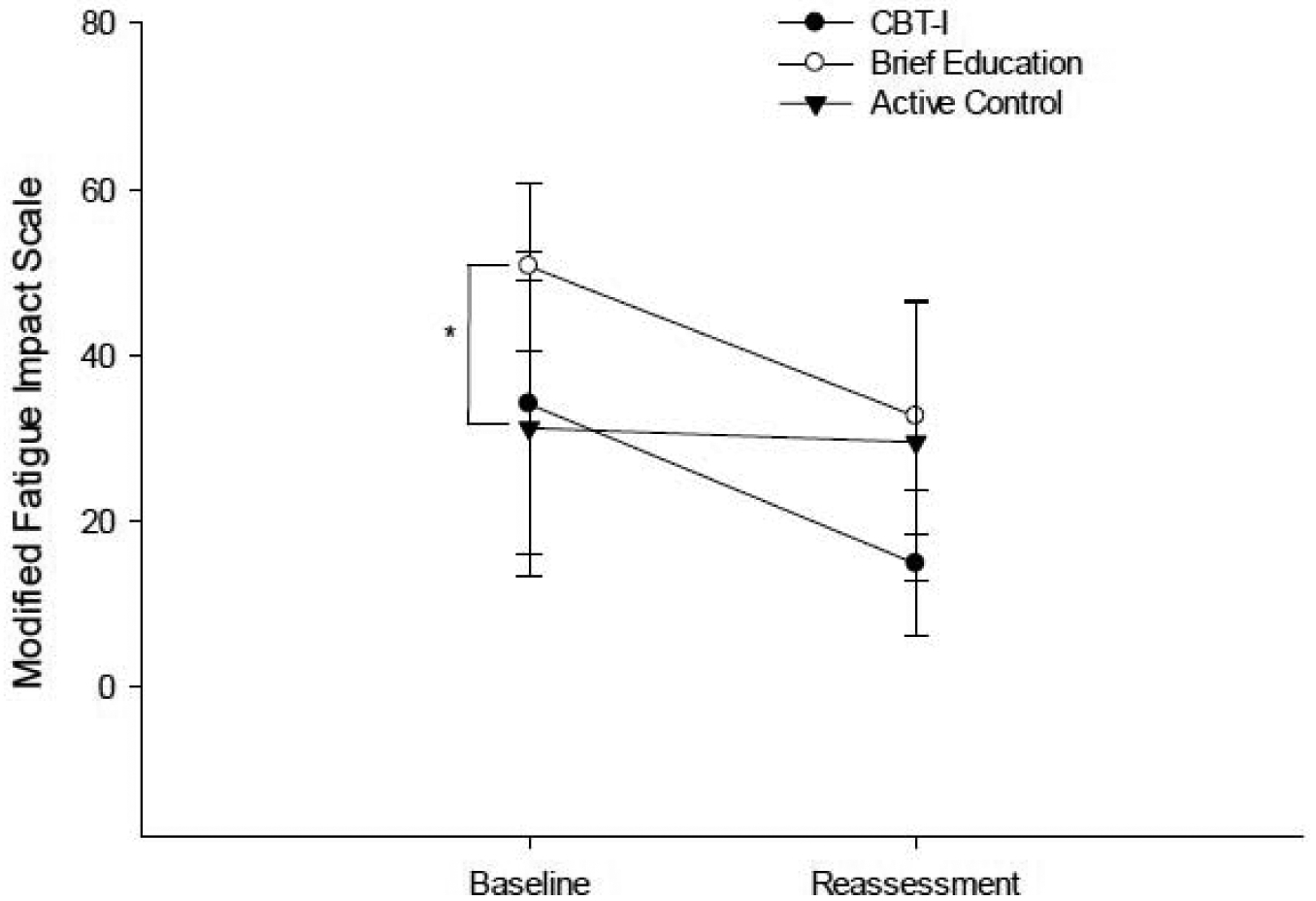
Performance on the Modified Fatigue Impact Scale at baseline and post-intervention reassessment. *indicates significant group difference at baseline.
Only the CBT-I group showed a significant moderate reduction on the FSS (Table 2; p= 0.043; ES = 0.524; Figure 5). There were no between group differences in magnitude of change on the FSS (Table 3).
Figure 5.
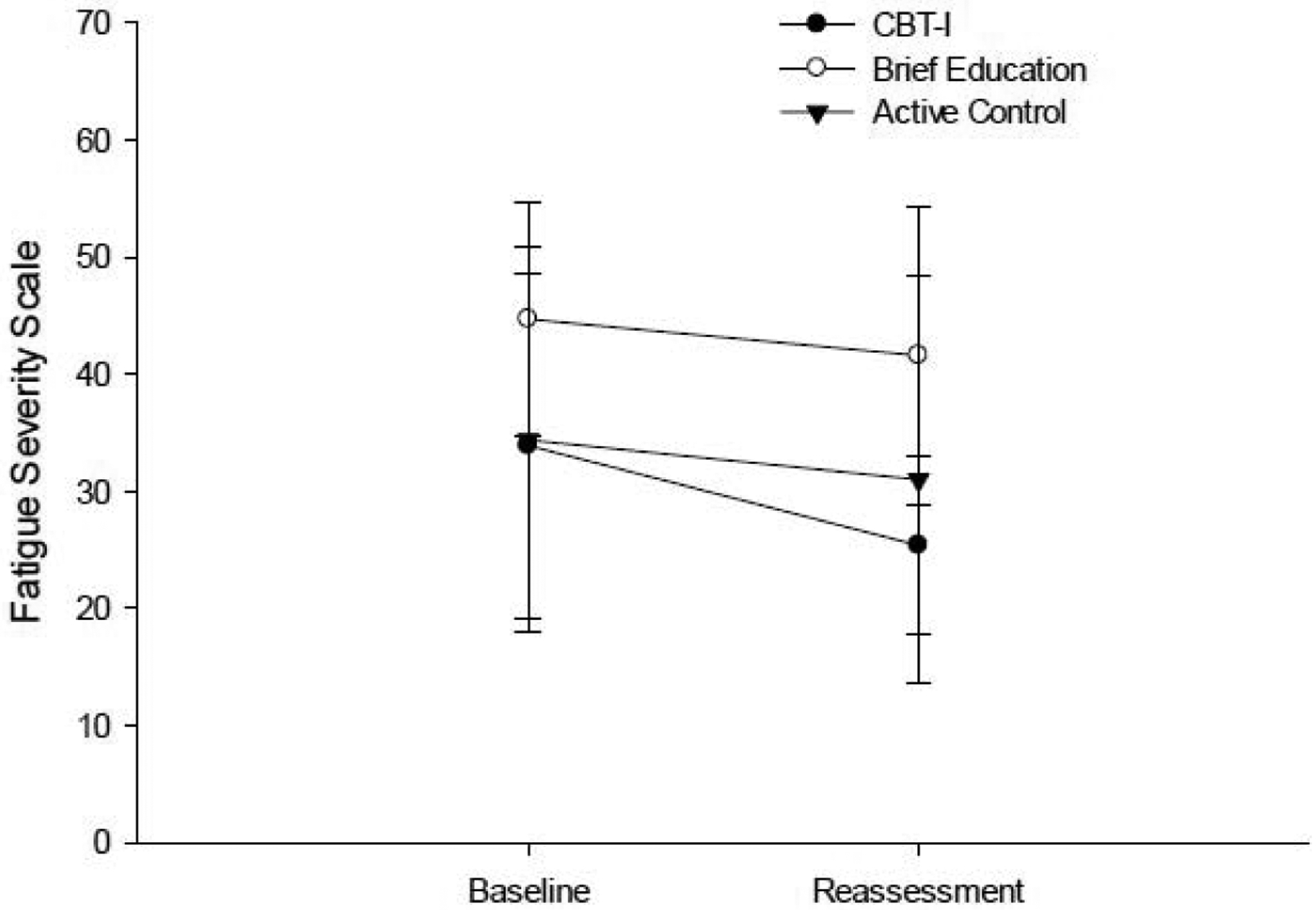
Performance on the Fatigue Severity Scale at baseline and post-intervention reassessment.
Treatment Effect: Secondary outcome measures
The CBT-I and BE group showed a significant increase on the SSE (Table 2; Figure 6) and decrease on the PHQ-9 (Table 2; Figure 7), but only the CBT-I group showed a large magnitude of change on the SSE (ES = 1.221) and PHQ-9 (ES = 0.798). There was a significant difference in magnitude of change on the SSE and PHQ-9 between the CBT-I group and the AC group (Table 3; SSE p= 0.014; PHQ-9 p = 0.007), but not between the CBT-I group and the BE group (Table 3; SSE p= 0.195; PHQ-9 p = 0.763).
Figure 6.
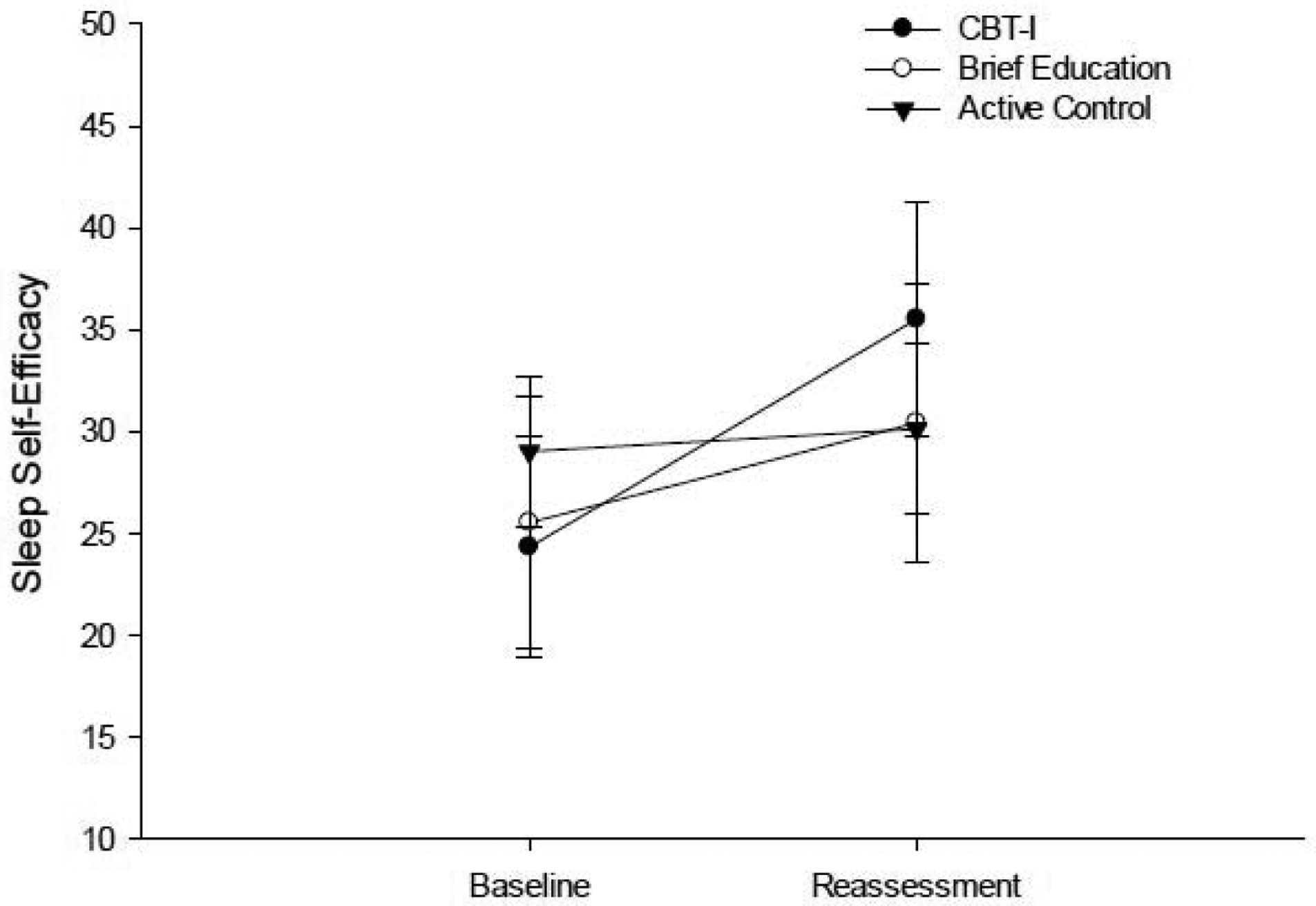
Performance on the SSE Scale at baseline and post-intervention reassessment.
Figure 7.
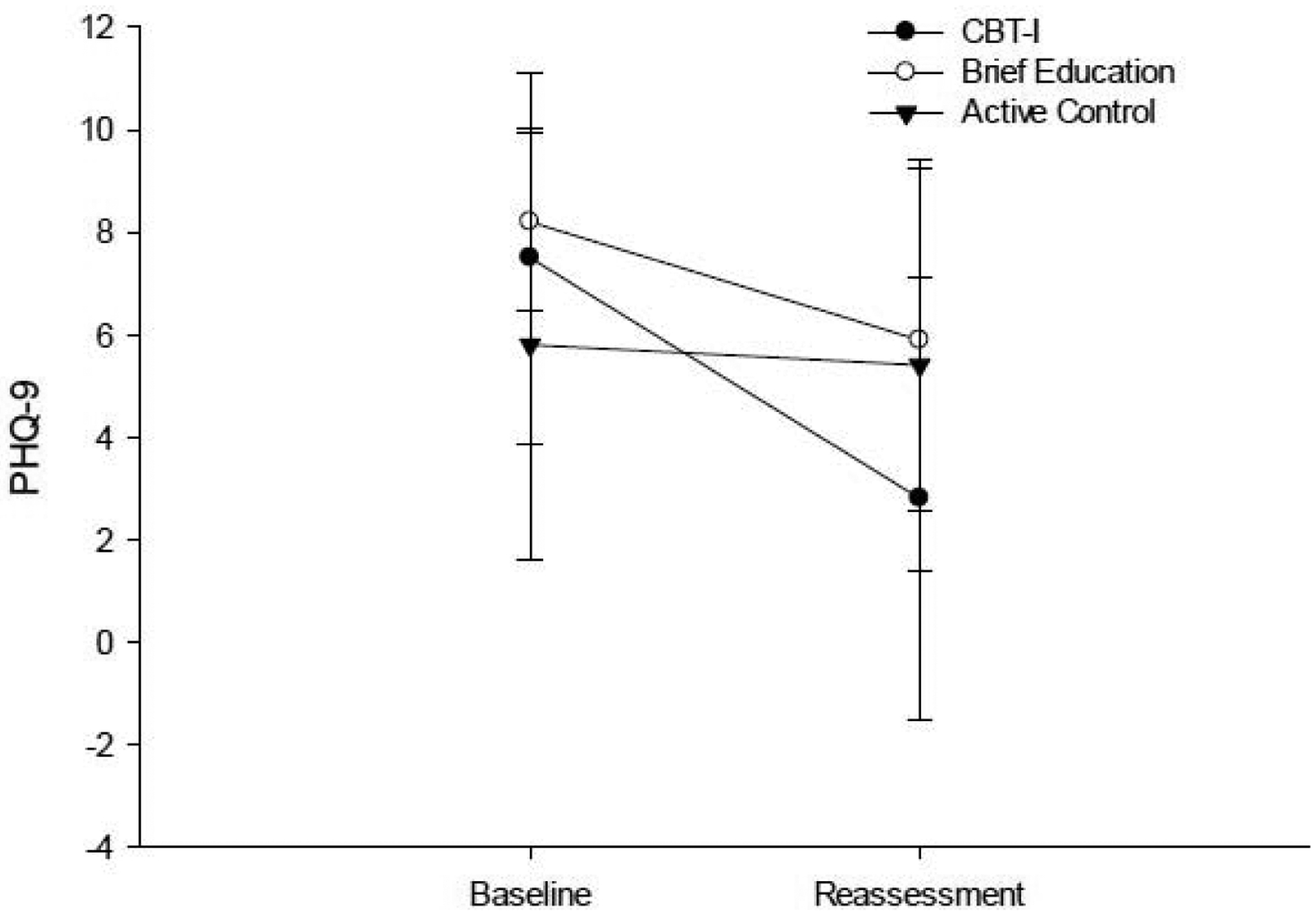
Performance on the PHQ-9 at baseline and post-intervention reassessment.
Only the CBT-I group showed a significant moderate reduction on the GAD-7 (Figure 8; Table 2; ES = 0.549). There were no between group differences in magnitude of change on the GAD-7 (Table 3).
Figure 8.
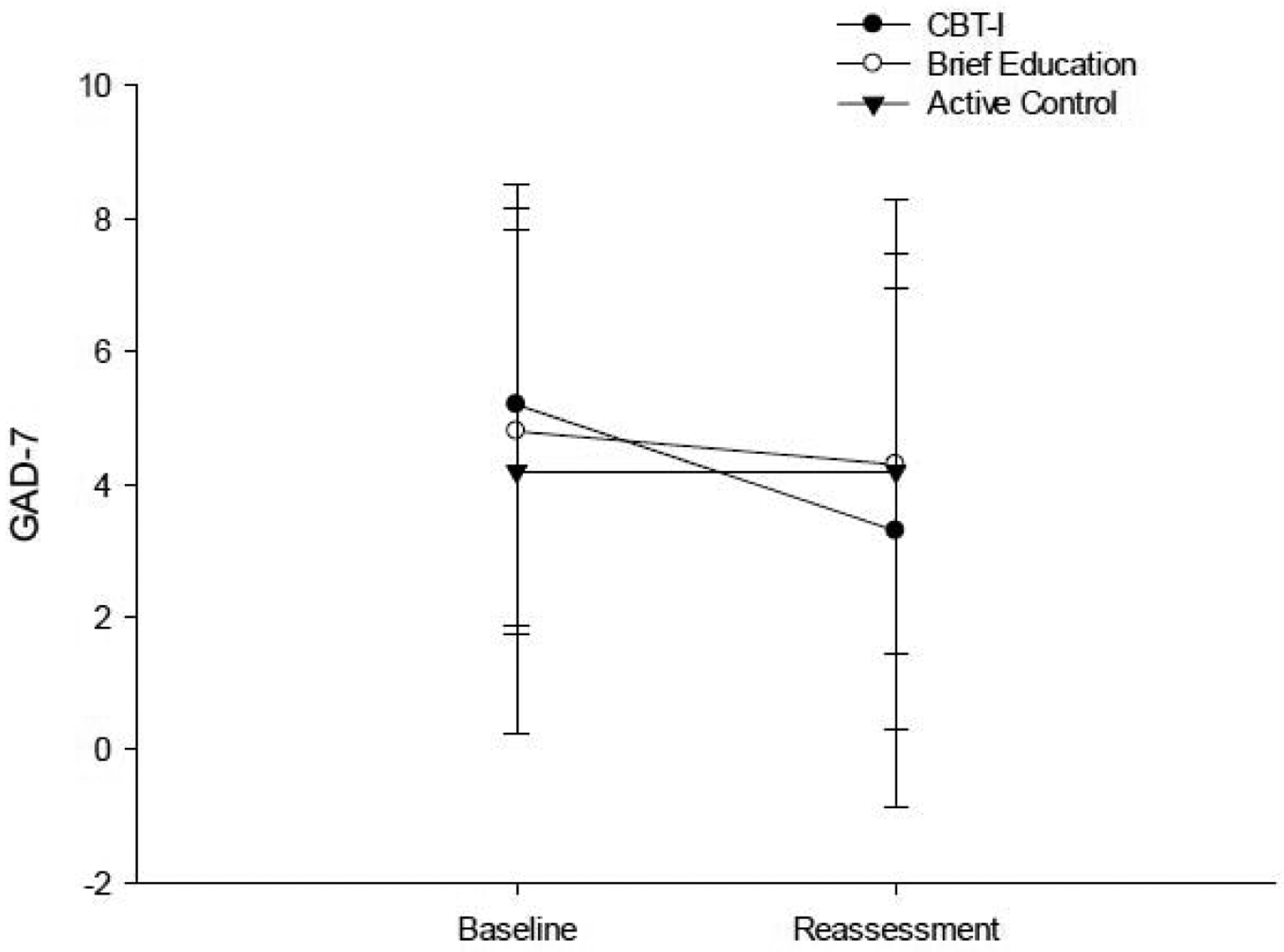
Performance on the GAD-7 at baseline and post-intervention reassessment.
Discussion
This study demonstrates for the first time that CBT-I in individuals with MS is feasible with high retention and adherence rate and low attrition rate. The results indicate that CBT-I may be an efficacious treatment for insomnia in individuals with MS. All three groups experienced a large magnitude of improvement in insomnia symptoms, and the CBT-I and brief education groups experienced a large magnitude of improvement in sleep quality and fatigue. Only the CBT-I group demonstrated a large magnitude of improvement in sleep self-efficacy and depression symptoms.
This study extends the promising results from the retrospective study conducted by Clancy et al.20 that found the majority of indivdiuals with MS who participated in a CBT-I intervention reported a reduction in insomnia, depression, and fatigue, and a case study in which an individual with MS experienced an improvement in sleep quality, life satisfaction, anxiety, and depression, and was able to eliminate sleep medication.21 It is particularly encouraging that while insomnia severity and sleep quality were improved for the CBT-I group in our study, comorbid symptoms including fatigue, depression, and anxiety were also improved, and sleep self-efficacy significantly increased. Given the association between sleep quality and fatigue, depression, and anxiety, it is possible that reducing insomnia symptoms and improving sleep quality had a direct impact on the improvement in fatigue, depression, and anxiety. While it is unclear the mechanism(s) underlying these improvements, it is possible that improvement in sleep self-efficacy contributed. Future studies designed to address mechanism of improvement are needed.
CBT-I is recommended as the initial approach to treatment of chronic insomnia32 and has been used shown to be an effective intervention for insomnia in individuals with a variety of comorbid conditions including chronic pain, cancer, depression, and PTSD (see review by Vitiello et al.33). However, it does not appear prescribing CBT-I for people with MS is mainstream, perhaps due to lack of evidence to support the use of CBT-I specifically in this population. A study by Braley et al.34 found that nearly half of individuals with MS reported occasional to frequent use of hypnotics to aid sleeping, and a quarter reported use of over-the-counter (OTC) hypnotics. Furthermore, use of an OTC hypnotic was a strong independent predictor of fatigue in that sample of nearly 200 individuals with MS.34 This is particularly concerning because hypnotics have been associated with increased daytime sleepiness, increased risk of falls and balance disturbances as well as tolerance and addition.32 Because CBT-I when conducted appropriately has minimal side effects15, this non-pharmacological intervention appears to have promising treatment effects in individuals with MS. One limitation of this treatment is the time-consuming nature of the treatment and the need for in-person meetings. An on-line intervention could be explored to reduce the problems of travel and reduce the time commitment while still allowing for an effective personal treatment.
One frequently cited issue with CBT-I is the limited number of providers trained to provide the intervention.35 Considering the brief education group had a significant improvement in insomnia severity, sleep quality, and fatigue, depression, and sleep self-efficacy, perhaps a “stepped-care” approach warrants consideration. In this model, tailored sleep education would be provided initially by a sufficiently trained person and treatment would be “stepped-up” for those who do not benefit as indicated. Clinical application of this model would require further study, but it provides an innovative method of addressing the high prevalence of insomnia symptoms in individuals with MS with the limited number of CBT-I providers.
It is interesting that the active control group experienced a significant, large improvement in insomnia severity despite receiving no intervention directed at improving insomnia symptoms or education regarding methods to improve their sleep. It would be unlikely that the gentle stretching and playing games 1 hour/week for 6 weeks would have a direct impact on the cognitive or behavioral factors that frequently perpetuate insomnia symptoms (i.e. irregular sleep schedule, spending time in bed not sleep, napping, negative thoughts regarding sleep). It seems possible that because the participants knew they were participating in a study designed to assess insomnia treatments, they had expectations that their insomnia symptoms would improve. Indeed, the placebo-effect is a well-reported phenomenon.36 However, despite the active control experiencing a significant, large improvement in insomnia symptoms, the CBT-I group and brief education group experienced a reduction in insomnia symptoms that was 2–3x the magnitude of the reduction experienced by the active control group. Therefore, the improvements experienced by the CBT-I and brief education cannot be explained by placebo-effect alone. It is also possible that being on a schedule to attend the control sessions served as a zeitgeber for individuals in the active control group and perhaps contributed to regulating their circadian rhythm.
A limitation of this study is the small sample size which limits the interpretation of the results but sets the basis for future studies in this area. Another limitation is that almost all participants were White, non-Hispanic, and female, which limits the generalizability of the results. This study also only included individuals with MS with minimal to moderate depression and anxiety. Future studies investigating the efficacy of CBT-I on insomnia and comorbid symptoms should use a control group matched for contact with research personnel and consider a wider range of inclusion and exclusion criteria to enhance generalizability.
In conclusion, this is the first study to prospectively demonstrates that CBT-I is feasible in people with MS and produces promising improvements in insomnia severity, sleep quality, sleep self-efficacy and comorbid symptoms of fatigue, depression, and anxiety. Future studies are needed to determine mechanisms for these improvements and expand the scope of individuals with MS who may benefit from CBT-I. Furthermore, considering the moderate to large improvements experienced by the brief education group and the limited number of CBT-I providers, a stepped-care approach warrants consideration.
Highlights.
CBT-I in individuals with MS is feasible with high retention and adherence rate.
CBT-I may be an efficacious treatment for insomnia in individuals with MS.
CBT-I may also be an efficacious treatment of fatigue, depression, and anxiety.
A stepped-care approach to treating insomnia warrants consideration.
Acknowledgements
We would like to acknowledge and thank our participants. We would also like to thank the National MS Society for their assistance with recruitment, and Samantha D’Silva, SPT, Stacy Coffyn, SPT, Alexandra Wileman, SPT, and Brock Watson, SPT for their assistance with the study.
Funding
This study was funded in part by the NMSS Pilot Grant (PP-1706-27878) awarded to CS, internal funds provided by the KUMC School of Health Professions and the Dept. of Physical Therapy and Rehabilitation Science awarded to CS, and by an NIH Clinical and Translational Science Award grant (UL1 TR000001, formerly UL1RR033179) awarded to the University of Kansas Medical Center. Its content are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Conflicting Interests
Catherine F Siengsukon: Owner and CEO of Sleep Health Education, LLC. Dr. Siengsukon has received funding from the National MS Society and the National Institutes of Health.
Mohammed Alshehri: no conflicts to report
Cierra Williams: no conflicts to report
Michelle Drerup: Dr. Drerup is the creator of Go! To Sleep and provides support for the web-based CBT-I program. She receives salary support from the Wellness Institute at the Cleveland Clinic but does not receive financial incentive for sale of the web-based CBT-I program.
Sharon Lynch: Dr. Lynch has participated in multi-center clinical trials in MS funded by Biogen, Genzyme, Teva, Sanofi, Novartis, Roche, NIH, NMSS, Acorda, Vaccinex, Adamas, Mallinkrodt and MedDay, and Actelion.
References
- 1.Siengsukon C, Alshehri M, Aldughmi M. Self-report sleep quality combined with sleep time variability distinguishes differences in fatigue, anxiety, and depression in individuals with multiple sclerosis: A secondary analysis. Multiple sclerosis journal - experimental, translational and clinical. 2018;4(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braley TJ, Segal BM, Chervin RD. Underrecognition of Sleep Disorders in Patients with Multiple Sclerosis. Journal of Clinical Sleep Medicine. 2015;11(1):81–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leonavicius R, Adomaitiene V. Features of sleep disturbances in multiple sclerosis patients. Psychiatria Danubina. 2014;26(3):249–255. [PubMed] [Google Scholar]
- 4.Medicine AAoS. International classification of sleep disorders, revised: Diagnostic and coding manual. Chicago, IL: American Academy of Sleep Medicine; 2001. [Google Scholar]
- 5.Taphoorn M, Van Someren E, Snoek F, et al. Fatigue, sleep disturbances and circadian rhythm in multiple sclerosis. Journal of neurology. 1993;240(7):446–448. [DOI] [PubMed] [Google Scholar]
- 6.Fleming WE PC. Sleep disorders in multiple sclerosis. Semin Neurol. 2005(25(1)): 64–68. [DOI] [PubMed] [Google Scholar]
- 7.Braley TJ, Chervin RD. Fatigue in multiple sclerosis: mechanisms, evaluation, and treatment. Sleep. 2010;33(8):1061–1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krupp LB, Alvarez LA, LaRocca NG, Scheinberg LC. Fatigue in multiple sclerosis. Archives of neurology. 1988;45(4):435–437. [DOI] [PubMed] [Google Scholar]
- 9.Glanz BI, Degano IR, Rintell DJ, Chitnis T, Weiner HL, Healy BC. Work productivity in relapsing multiple sclerosis: associations with disability, depression, fatigue, anxiety, cognition, and health-related quality of life. Value in health: the journal of the International Society for Pharmacoeconomics and Outcomes Research. 2012;15(8):1029–1035. [DOI] [PubMed] [Google Scholar]
- 10.Blaney BE, Lowe-Strong A. The impact of fatigue on communication in multiple sclerosis. The insider’s perspective. Disability and rehabilitation. 2009;31(3):170–180. [DOI] [PubMed] [Google Scholar]
- 11.Veauthier C, Paul F. Fatigue in multiple sclerosis: which patient should be referred to a sleep specialist? Multiple sclerosis (Houndmills, Basingstoke, England). 2012;18(2):248–249. [DOI] [PubMed] [Google Scholar]
- 12.Suarez EC. Self-reported symptoms of sleep disturbance and inflammation, coagulation, insulin resistance and psychosocial distress: evidence for gender disparity. Brain, behavior, and immunity. 2008;22(6):960–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veauthier C, Gaede G, Radbruch H, Gottschalk S, Wernecke K- D, Paul F. Treatment of sleep disorders may improve fatigue in multiple sclerosis. Clinical neurology and neurosurgery. 2013;115(9):1826–1830. [DOI] [PubMed] [Google Scholar]
- 14.Wu JQ, Appleman ER, Salazar RD, Ong JC. Cognitive Behavioral Therapy for Insomnia Comorbid With Psychiatric and Medical Conditions: A Meta-analysis. JAMA Intern Med. 2015;175(9):1461–1472. [DOI] [PubMed] [Google Scholar]
- 15.Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive Behavioral Therapy for Chronic Insomnia: A Systematic Review and Meta-analysis. Ann Intern Med. 2015;163(3):191–204. [DOI] [PubMed] [Google Scholar]
- 16.Wang MY, Wang SY, Tsai PS. Cognitive behavioural therapy for primary insomnia: a systematic review. Journal of advanced nursing. 2005;50(5):553–564. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell MD, Gehrman P, Perlis M, Umscheid CA. Comparative effectiveness of cognitive behavioral therapy for insomnia: a systematic review. BMC family practice. 2012;13:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castronovo V, Galbiati A, Sforza M, et al. Long-term clinical effect of group cognitive behavioral therapy for insomnia: a case series study. Sleep Med. 2018;47:54–59. [DOI] [PubMed] [Google Scholar]
- 19.Geiger-Brown JM, Rogers VE, Liu W, Ludeman EM, Downton KD, Diaz-Abad M. Cognitive behavioral therapy in persons with comorbid insomnia: A meta-analysis. Sleep Med Rev. 2015;23:54–67. [DOI] [PubMed] [Google Scholar]
- 20.Clancy M, Drerup M, Sullivan AB. Outcomes of Cognitive-Behavioral Treatment for Insomnia on Insomnia, Depression, and Fatigue for Individuals with Multiple Sclerosis: A Case Series. International journal of MS care. 2015;17(6):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Majendie CMA, Dysch L, Carrigan N. Cognitive Behavioral Therapy for Insomnia (CBT-I) for an Adult With Multiple Sclerosis. Clinical Case Studies. 2017;16(2):115–131. [Google Scholar]
- 22.Morin CM, Belleville G, Belanger L, Ivers H. The Insomnia Severity Index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001;16(9):606–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. [DOI] [PubMed] [Google Scholar]
- 25.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Archives of neurology. 1989;46(10):1121–1123. [DOI] [PubMed] [Google Scholar]
- 26.Ritvo PG, Fischer JS, Miller DM, Andrews H, Paty DW, LaRocca NG. MSQLI: Multiple Sclerosis Quality of Life Inventory: A User’s Manual. New York, NY: National Multiple Sclerosis Society; 1997. [Google Scholar]
- 27.Swinson RP. The GAD-7 scale was accurate for diagnosing generalised anxiety disorder. Evidence-based medicine. 2006;11(6):184. [DOI] [PubMed] [Google Scholar]
- 28.Lacks P Behavioral Treatment for Persistant Insomnia. New York, NY: Pergamon Press; 1987. [Google Scholar]
- 29.Perlis M, Jungquist C, Smith M, Posner D. Cognitive Behavioral Treatment of Insomnia: A Session-by-Session Guide. New York, NY: Springer; 2008. [Google Scholar]
- 30.Spielman AJ, Caruso LS, Glovinsky PB. A behavioral perspective on insomnia treatment. Psychiatr Clin North Am. 1987;10(4):541–553. [PubMed] [Google Scholar]
- 31.Cohen J Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 32.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. Journal of clinical sleep medicine: JCSM: official publication of the American Academy of Sleep Medicine. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 33.Vitiello MV, McCurry SM, Rybarczyk BD. The future of cognitive behavioral therapy for insomnia: what important research remains to be done? Journal of clinical psychology. 2013;69(10):1013–1021. [DOI] [PubMed] [Google Scholar]
- 34.Braley TJ, Segal BM, Chervin RD. Hypnotic use and fatigue in multiple sclerosis. Sleep Medicine. 2015;16(1):131–137. [DOI] [PubMed] [Google Scholar]
- 35.Perlis ML, Smith MT. How can we make CBT-I and other BSM services widely available? Journal of Clinical Sleep Medicine. 2008;4(1):11–13. [PMC free article] [PubMed] [Google Scholar]
- 36.Beecher HK. The Powerful Placebo. Jama-J Am Med Assoc. 1955;159(17):1602–1606. [DOI] [PubMed] [Google Scholar]


