FIGURE 1.
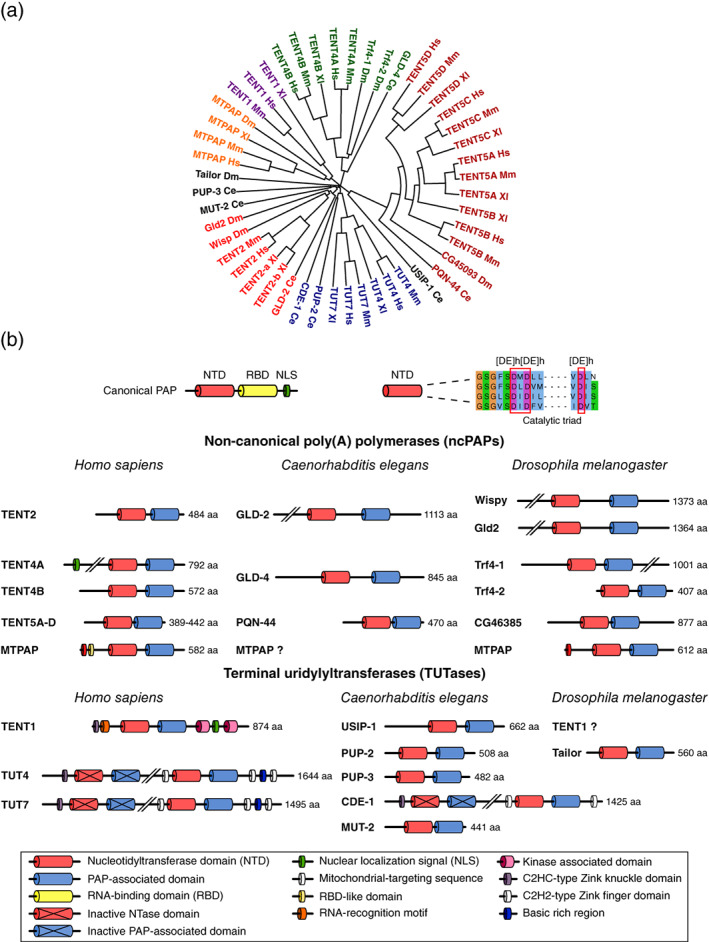
(a) A phylogenetic relationship of TENTs from vertebrates, worms, and fruit fly. (b) Schematic representation of terminal nucleotidyltransferases (TENTs) from the human, worm, and fruit fly genomes. Canonical nuclear poly(A) polymerase (PAP) is a highly evolutionarily conserved PAP. It comprises an N‐terminal nucleotidyltransferase (NTase) catalytic domain, a central RNA‐binding domain (RBD), and a C‐terminal domain with a nuclear localization signal (NLS). Catalytic activity relies on the highly conserved aspartate triad ([DE]h[DE]h [DE]h in the NTase domain. All TENTs share similar catalytic domain architecture but lack the RBD. The domain architecture of TENTs is diverse, and all additional domains and regions are indicated for each TENT. The length (aa) of each enzyme is indicated on the right
