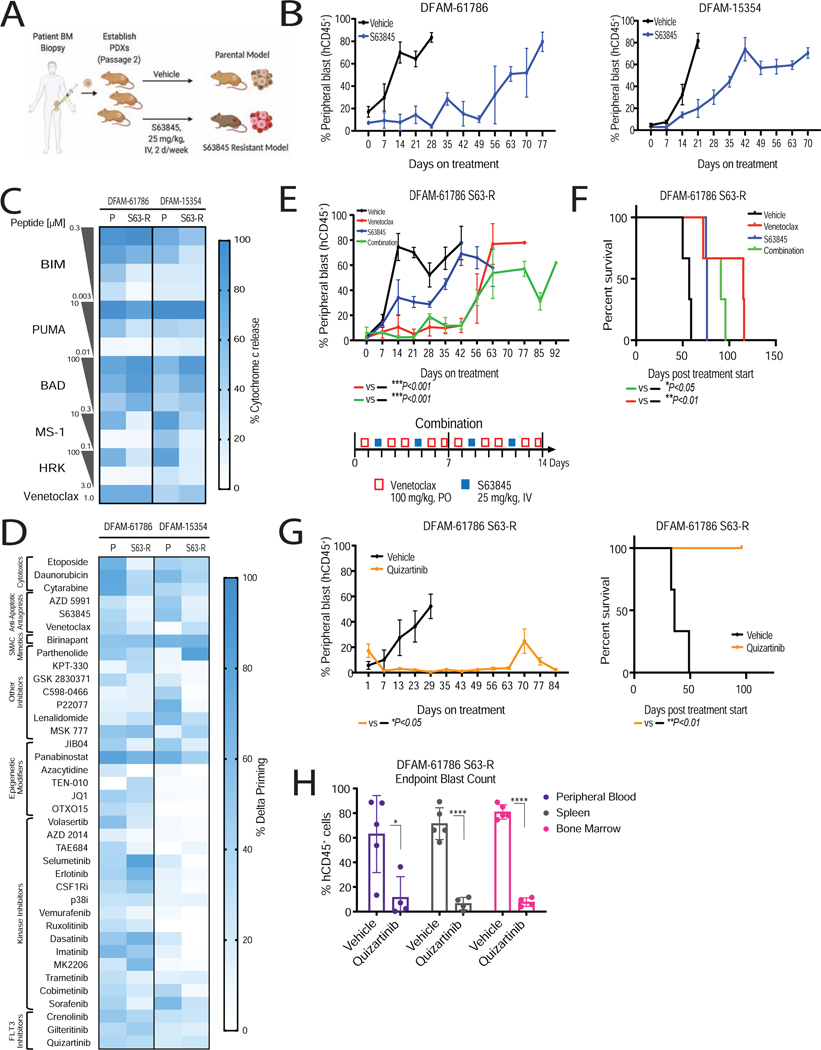
Figure 8: Dynamic BH3 profiling identified BCL-2 antagonism and FLT3 inhibition as an effective strategy in S63845 resistant settings.
(A) Schematic of modeling of in vivo S63845 resistance in AML PDXs.
(B) Leukemic burden in indicated PDX models treated with S63845 (25 mg/kg, IV, 2 days/week) or vehicle treatment. Mean±SEM, n=5 mice.
(C) Comparison of baseline mitochondrial priming of parental (P) and S63845 resistant (R) PDXs, determined by BH3 profiling (n=3 mice/group).
(D) Heatmap of delta priming responses in the myeloblasts derived from parental and S63845-resistant PDXs at 16 h drug treatment, determined via DBP. Each entry reflects 3 independent mice as biological replicates of 2 technical replicates each.
(E, F) S63845 resistant myeloblasts from DFAM-61786 were transplanted into NSG mice and assigned to treatment arms. (E) % hCD45+ peripheral blast reduction in response to venetoclax (100 mg/kg, PO, 5 days/week), S63845 (25 mg/kg, IV, 2 days/week), and combination treatment. Mean±SD, n=5 mice; ***P<0.001 one-way ANOVA. (F) Corresponding Kaplan-Meier survival curve. *P<0.05, **P<0.01, log-rank test.
(G) % hCD45+ peripheral blast reduction and corresponding Kaplan-Meier survival curve in response to quizartinib (10 mg/kg, IP, 5 days/week). Mean±SD, n=5 mice.
(H) % hCD45+ blast reduction across different compartments. Mean±SD, n=5 mice; *P<0.05, **P<0.05, two-tailed student t-test.
See also Figure S8.