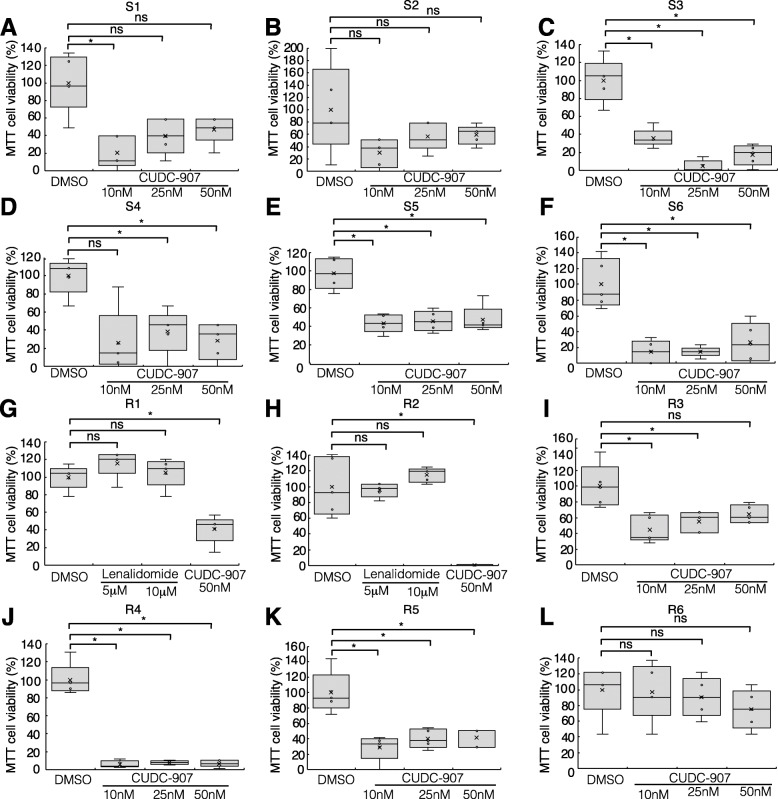
Fig. 8.
MTT proliferation assay were performed in primary cells from 12 MM patients. a–f MTT proliferation assays were performed in primary cells from six lenalidomide-sensitive MM patients. Cells were treated with 10–50 nM CUDC-907 for 48 h. g-l MTT proliferation assays were performed in primary cells from six lenalidomide-resistant MM patients. Cells were treated with 5 μM lenalidomide, 10 μM lenalidomide, or 10–50 nM CUDC-907 for 48 h. (*p < 0.05, **p < 0.01, “ns” indicates no significant difference.) Clinical parameters of MM patients are shown in Table 1