Figure.
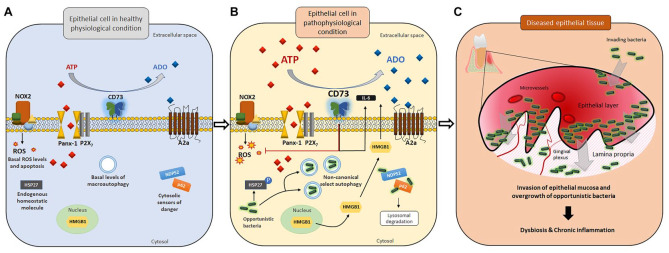
Disrupted homeostatic environment in gingival epithelial cells upon invasion by opportunistic bacteria may lead to bacterial dysbiosis in tissues. In gingival epithelial cells, various host defense machineries are in place to combat potential bacterial offenses. (A) At the physiologic level, a minimal amount of ATP is released to the extracellular space via the pannexin 1 (Panx-1) hemichannel without activating P2X7-mediated purinergic signaling and ROS-generating NADPH oxidase 2 (NOX2), resulting in a basal level of apoptosis. The equilibrium in extracellular purine concentration between ATP and adenosine is well balanced. Other danger signal molecules (e.g., HMGB1) and ubiquitination markers/cytosolic sensors of danger (including NDP52 and p62) remain mostly inactive. Antiapoptotic protein HSP27 is largely unphosphorylated. (B) Upon pathogenic bacterial insults, the extracellular ATP (eATP) release drastically increases, which then activates the P2X7 receptor and NOX2 as antibacterial responses. HMGB1, which requires high eATP levels as a second stimulus, becomes translocated from the nucleus to the cytosol and extracellular space. However, opportunistic bacteria such as Porphyromonas gingivalis can modulate multiple antibacterial host machineries (NOX2, P2X7, HMGB1, and ubiquitination marking) and stimulate probacterial host machineries (probacterial autophagy, CD73 activation, HSP27 phosphorylation, and A2a receptor signaling) for successful long-term colonization. (C) Opportunistic pathogens can invade host epithelial tissue and establish chronic colonization while penetrating deeper into the lamina propria. Uncontrolled growth of such pathogenic bacteria and further host tissue invasion lead to bacterial dysbiosis in the epithelia, ultimately initiating the formation of chronic inflammation and diseased epithelial tissues with increased formation of microvasculature.