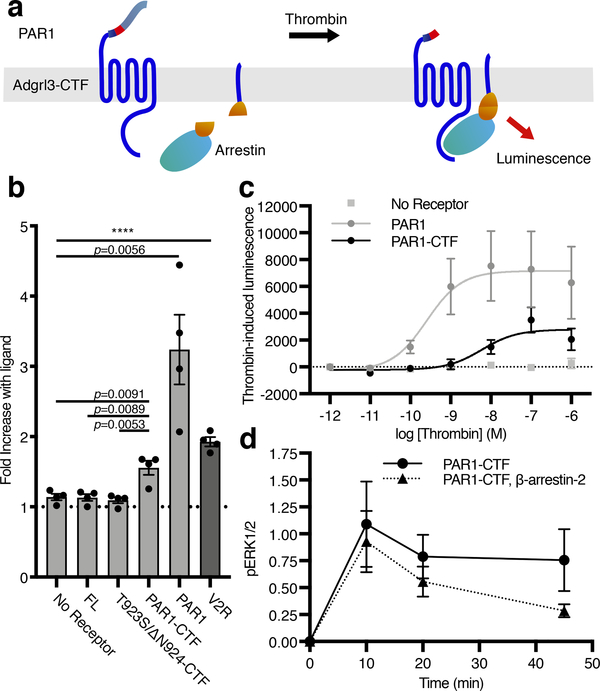
Figure 4. Adgrl3 recruits β-arrestin to the plasma membrane in living cells.
(a) Cartoon outlining the principle of the split complementation luminescence β-arrestin assay. After receptor activation (and potentially phosphorylation) β-arrestin-C-nluc is recruited to the membrane to complement a membrane anchored N-nluc. Upon β-arrestin-translocation and reconstitution of a functional nluc, a luminescence signal is produced. (b) β-Arrestin-2 membrane-recruitment complementation assay for negative controls of empty vector, FL, and T923S/ΔN924-CTF constructs as well as for PAR1-CTF, PAR1 in response to 1 μM thrombin, and the vasopressin receptor V2R in response to 1 uM Vasopressin (AVP), which is a high affinity β-arrestin binder. Data are shown as fold over control (buffer). Unpaired two-tailed t-tests were performed to determine statistical significance between the PAR1-CTF and ‘No receptor’, FL and T923S/ΔN924-CTF constructs, as well as ‘No receptor’ and controls PAR1 and the vasopressin receptor V2R. (c) Dose-response curves for a negative control (empty vector), PAR1-CTF, and PAR1. Bars in (b) and data points in (c) are presented as mean ±SEM from 4 independent experimental replicates. (d) β-arrestin-2 decreased PAR1-CTF ERK1/2 phosphorylation. HEK Δβarr1/2 cells were transfected with PAR1-CTF, or PAR1-CTF with β-arrestin-2. After 48 hr, the cells were acutely activated with 1 μM thrombin. The level of phospho-ERK1/2 was normalized to total ERK and the baseline at 0 min was subtracted to produce the time-dependent change in pERK1/2. Data in (d) represent mean ±SEM from 3 (PAR1-CTF) and 4 (PAR1-CTF, β-arrestin-2) independent experimental replicates.