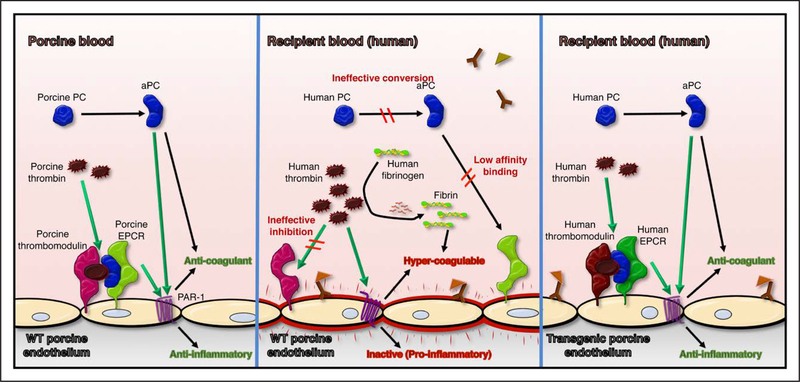
Figure 2. Dysregulated coagulation with porcine endothelium exposed to human blood.
Relative to physiological regulation of thrombosis (left), porcine endothelium exposed to human blood is activated by binding of anti-pig antibodies, creating a prothrombotic environment (middle). Physiologically inappropriate amplification of blood clotting is contributed to by ineffective neutralization of human thrombin by porcine thrombomodulin, inefficient conversion of protein C (PC) to activated PC (aPC) by thrombin-thrombomodulin complex, and low-affinity binding of human aPC to porcine endothelial protein C receptor (EPCR), which in turn leads to inefficient thrombin degradation and reduced cytoprotective signaling through endothelial cell proteinase-activated receptor 1 (PAR-1). These molecular incompatibilities between species are addressed by expression of human thromboregulatory proteins, including human thrombomodulin and human EPCR (right), as well as human tissue factor pathway inhibitor (not illustrated). WT indicates wild-type.