Fig. 1.
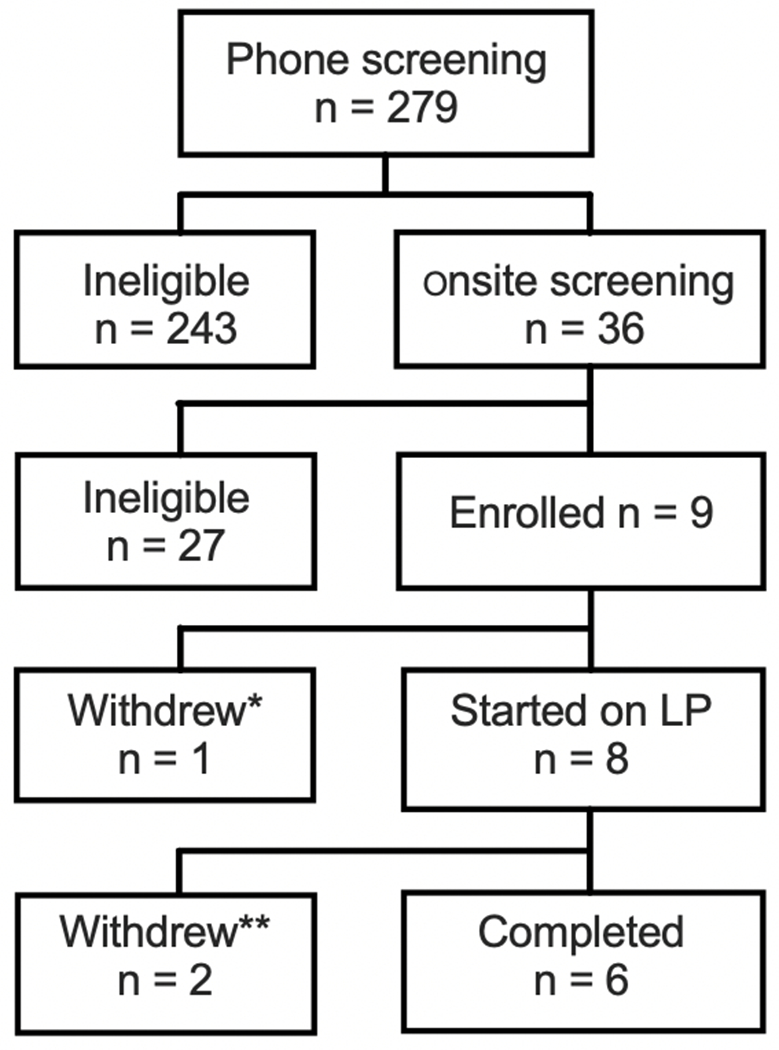
Consort flow diagram of subject accrual into the trial. * The patient withdrew prior to starting on study medication due to relocation to another state. ** withdrew by MD due to adverse event deemed not related to study medication.

Consort flow diagram of subject accrual into the trial. * The patient withdrew prior to starting on study medication due to relocation to another state. ** withdrew by MD due to adverse event deemed not related to study medication.