Table 1.
Reactions of triyne 8 with several C,N-diaryl imines.
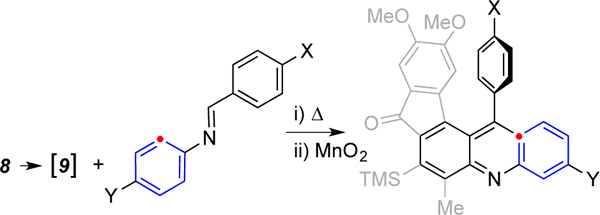 | ||
|---|---|---|
| entry | imine | product [step ia: yield%, step iib: yield%] |
| 1 | 2a, X = H, Y = H | 12a [step ia: 98%, step iib: 68%] |
| 2 | 2b, X = NO2, Y = H | 12b [step ia: 68%, step iib: 90%] |
| 3 | 2c, X = OMe, Y = H | 12c [step ia: 78%, step iib: 86%] |
| 4 | 2d, X = H, Y = NO2 | 12d [step ia: 62%, step iib: 82%] |
| 5 | 2e, X = H, Y = OMe | 12e [step ia: 100%, step iib: 97%] |
| 6 | 2f, X = NO2, Y = NO2 | 12f [step ia: 19%, step iib: 56%] |
| 7 | 2g, X = OMe, Y = OMe | 12g [step ia: 88%, step iib: 89%] |
yield of the 1,4-dihydroacridine from the HDDA reaction
yield of the acridine adduct following MnO2 treatment
