Figure 5. A unifying epigenetic mechanism of the effects of alcohol consumption on different stages of the addiction cycle that lead to alcohol use disorder.
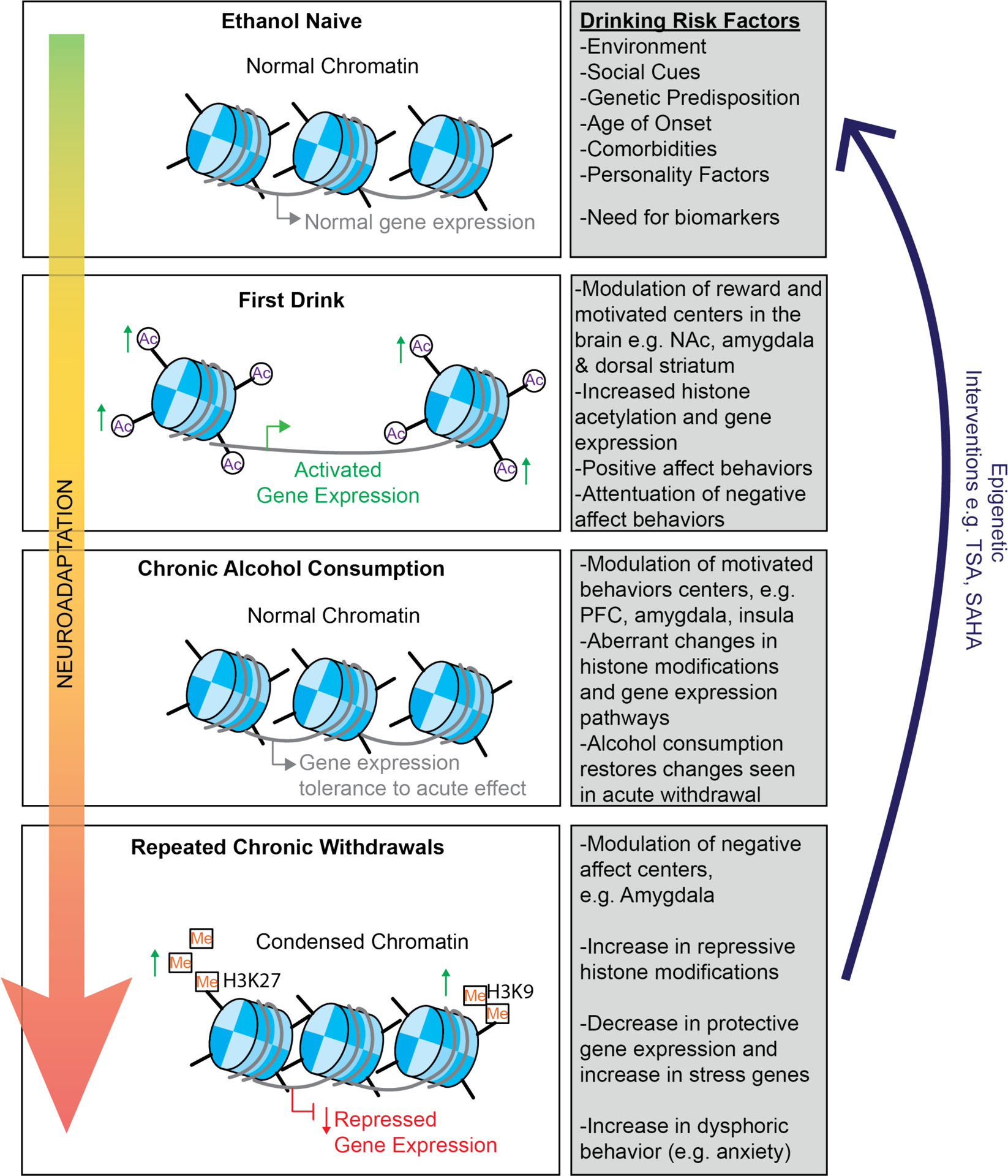
Preexisting risk factors can lead to the initiation of alcohol consumption. The first drink or alcohol experience modulates reward and motivational centers of the brain such as the nucleus accumbens (NAc), dorsal striatum, and amygdala which underlie the rewarding or positive affect of alcohol consumption and attenuate negative affect. These are associated with increased histone acetylation and gene expression. During a second stage, the move to chronic alcohol consumption causes changes in motivation and executive centers of the brain such as the insula and there is tolerance to the rewarding effects of ethanol and the epigenetic effects such as increased gene activation. In the third stage, repeated chronic consumption and withdrawal activates negative affect centers such as the amygdala and the bed nucleus of the stria terminalis (BNST) and causes decreased histone acetylation, increased repressive histone methylation and repressed gene expression which correspond with an increase in dysphoric behaviors such as anxiety. Acute ethanol challenge can restore both the epigenetic changes and behavioral changes to a more baseline levels. Importantly, these effects are also observed when ethanol is consumed during critical periods of development, such as during gestation or adolescence. Treatment with epigenetic drugs or genetic strategies can restore normal epigenetic pathways and functions and corresponding behaviors. This ultimately suggests that drugs that work on an epigenetic level could be useful for the treatment of alcohol use disorder.
