Figure 2.
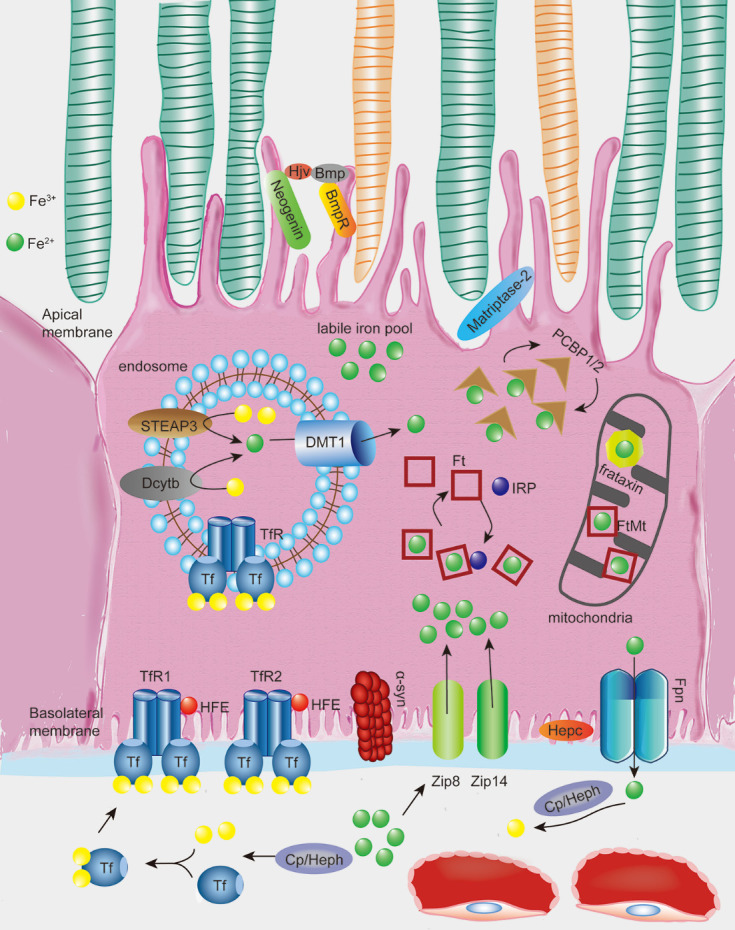
Illustration of the processes of iron uptake, storage, and efflux in the retinal pigment epithelium (RPE) cells. Two Fe3+atoms oxidized from Fe2+ by ferroxidase hephaestin (Heph) or ceruloplasmin (Cp) bind to the iron transport protein transferrin (Tf). Tf then binds to Tf receptor 1/2 (TfR1/2) in the basolateral membrane of RPE, modulated by HFE. In some conditions, retinal non-Tf bound iron import may be absorbed by Zip8 and/or Zip14 independent of the canonical Tf-TfR pathway without being oxidized. A ferrireductase, α-synuclein (α-syn) expressed in RPE cells can facilitate the uptake of Tf-bound iron but not non-Tf bound iron. Once inside cells, Fe3+ dissociates from the Tf-TfR complex in acidified endosomes followed by reduction of Fe3+ to Fe2+ catalyzed by ferrireductases, such as Steap3 and Dcytb, then transported across the endosomal membrane into the cytoplasm by the ferrous iron transporter DMT1. Fe2+ may then be stored into Ft in the cytoplasm or FtMt in the mitochondria, both of which are regulated by iron regulatory proteins (IRPs). Fe2+ is released from Ft through Ft degradation and is selectively recognized by the cytosolic and mitochondrial Fe2+ chaperones PCBPs1/2 and frataxin, respectively, then eventually utilized by diverse Fe2+-dependent proteins. The remaining Fe2+ enters the labile iron pool as the source of active-redox iron. Fe2+ that is not utilized or stored by the cell is exported by the transmembrane protein Fpn through post-translational regulation by hepcidin (Hepc). Fpn is also regulated by HFE, which is located at the basolateral membrane and by hemojuvelin (Hjv) and matriptase2, which are located at the apical membrane of the RPE through their regulatory effect towards Hepc. Fe2+ efflux is subsequently oxidized by ferroxidase Heph or Cp to facilitate the next cycle of iron uptake.
