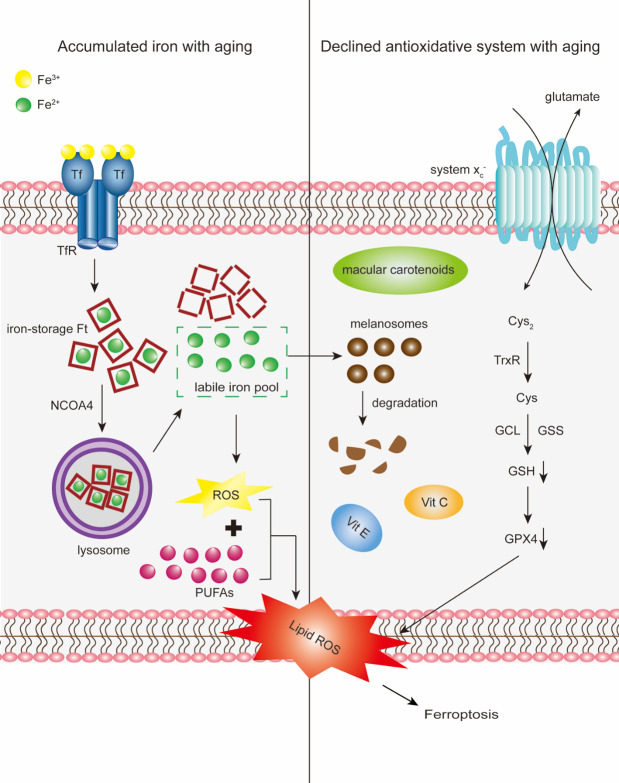
Figure 4.
Schematic of the proposed involvement of ferroptosis in the aging retina and AMD. Cargo receptor NCOA4 delivers iron-storage macromolecule Ft to the lysosomes, where Ft is then degraded. Fe2+released into the cytoplasm from degraded Ft constitutes the labile iron pool. With aging, accumulated iron exceeds the storage capacity of retinal cells, enters the labile iron pool and expands the redox-active iron pool. Fe2+ from the labile iron pool subsequently generates ROS via Fenton reactions. ROS further react with PUFAs to generate lipid-ROS and promote lipid peroxidation. Conversely, a decline in lipid antioxidants with aging, particularly GSH, alters the antioxidative capacity of retinal cells. Synthesis of GSH from glutamate, cysteine (Cys), and glycine occurs via two steps catalyzed by two ATP-dependent cytoplasmic enzymes namely glutamate-cysteine ligase (GCL) and glutathione synthetase (GSS). Intracellular GSH biosynthesis is dependent on the availability of Cys, the reduced form of cystine (Cys2) catalyzed by thioredoxin reductase (TrxR). Cys2 uptake is mainly mediated by the system xc-, the upstream determinant of ferroptosis. Decline in GSH with aging ultimately inactivates GPX4, the sole enzyme that reduces lipid hydroperoxides within biological membranes. Iron-mediated melanosome degradation reduces its ability to inhibit iron-induced lipid peroxidation. Iron accumulation and decline in lipid antioxidants, coordinatively aggravate age-related iron-induced lipid peroxidation, initiating ferroptosis.