Abstract
Epigenetic studies in animal models have demonstrated that diet affects gene regulation by altering methylation patterns. We interrogated methylomes in humans who have different sources of protein in their diet. We compared methylation of DNA isolated from buffy coat in 38 vegans, 41 pescatarians and 68 nonvegetarians. Methylation data were obtained using Infinium HumanMethylation450 arrays and analyzed using the Partek Genomic software. Differences in differentially methylated sites were small, though with the use of relaxed statistical tests we did identify diet-associated differences. To further test the validity of these observations, we performed separate and independent comparisons of the methylation differences between vegans and nonvegetarians, and between vegans and pescatarians. The detected differences were then examined to determine if they were enriched in specific pathways. Pathway analysis revealed enrichment of several specific processes, including homeobox transcription and glutamate transport. The detected differences in DNA methylation patterns between vegans, pescatarians, and nonvegetarians enabled us to identify 77 CpG sites that may be sensitive to diet and/or lifestyle, though high levels of individual-specific differences were also noted.
Keywords: differential DNA methylation, epigenetics, pathway analysis, vegan diet
1. Introduction
Vegetarianism, a diet characterized by abstinence from meats, poultry, and fish, has been reported to reduce risk factors for several chronic diseases, and, in particular, is associated with a lower incidence of and mortality from ischemic heart disease [1]. A recent systematic review and meta-analysis of observational analyses further provide evidence of the benefits of vegan diets (abstention from all flesh foods, dairy, and eggs) on cancer [2]. The inclusion of a healthy vegetarian diet in the 2015–2020 Dietary Guidelines for Americans [3] likely will increase vegetarianism as a dietary choice among all ages. Nevertheless, the processes and mechanisms that explain how diet may interact with gene regulation, epigenetics included, are largely unknown, though further understanding of these factors has the potential to significantly improve health and quality of life [4].
Recent advances regarding the association of diet and epigenetic changes have been summarized in a review by Sapienza and Issa [5]. Significant advances have been achieved in our understanding of the role of dietary components on epigenetic alterations using animal models. For example, it has been shown that both maternal methyl-donor supplementation and calorie restriction induces epigenetic changes in murine offspring [6,7], and that a maternal high fat diet affects the methylomes of neonatal offspring rats [8]. Studies on human subjects are much less available, largely due to difficulties in the collection of sufficient and appropriate samples, and often display inconsistent results [5]. However, epidemiological human studies have identified dietary factors that may explain epigenetic alterations, possibly including a transgenerational effect that depends on maternal food consumption [9]. Direct evidence of such dietary regulation of epigenetic changes was found by studying methylation patterns of several loci in children of individuals who were exposed to famine during World War II in the Netherlands [10].
In this present study, we applied comparative methylome analysis to characterize and compare the DNA methylation patterns in vegans, pescatarians and nonvegetarians. We then employed pathway analysis to assess whether there was gene enrichment in specific processes, as this would indicate nonrandom selection of genes. Performing these analyses independently for both the vegan/pescatarian and the vegan/nonvegetarian comparisons enabled us to strengthen our conclusion that diet can influence epigenetic patterns.
2. Materials and Methods
2.1. Subjects and Study Design
The Adventist Health Study-2 (AHS-2) is a prospective cohort of 96,592 Adventists in North America established between 2002 and 2007. Recruitment and selection methods have been reported previously in detail [11]. This study was performed by protocol #5130319 approved by the Institutional Review Board of Loma Linda University and all participants gave written consent at enrollment. From this collection, we selected a sample of 147 individuals with contrasting dietary patterns (vegan, pescatarian, and nonvegetarian), matched by gender and age, for these methylation studies: 38 vegans, 41 pescatarians and 68 nonvegetarians (omnivores).
2.2. Dietary Assessment
Nutrient and food intake were assessed by a previously validated food frequency questionnaire [12]. Dietary intake estimates were calculated using the product-sum method [13] where intake = sum[(weighted frequency of use of a food) × (weighted portion size consumed of that food) × (nutrient content in a standard serving size of that food)]. Dietary patterns were determined according to the reported frequency of intake of animal-based foods from the FFQ [14]. Specifically, vegans consumed eggs/dairy, fish, and all other meats never or rarely; lacto-ovo vegetarians consumed eggs/dairy 1 time/mo or more, but fish and all other meats less than 1 time/mo; pescatarians consumed fish 1 time/mo or more, but all other meats less than 1 time/mo; semi vegetarians consumed non-fish meats 1 time/mo or more, and all meats combined (fish included) 1 time/mo or more but no more than 1 time/wk; and last, nonvegetarians consumed non-fish meats 1 time/mo or more, and all meats combined (fish included) more than 1 time/wk.
2.3. Anthropometric and Lifestyle Measures
We measured body weight and height using standard protocols, and collected fasting blood samples at field clinics held in church halls. A questionnaire completed at baseline provided information on physical activity (min/week), sleep hours (h/day), perception of one’s own health (excellent, good, fair), prevalent cancer and CVD comorbidities, and cigarette smoking (never or ever).
2.4. Blood Collection
Fasting blood was obtained at field clinics, collected in heparin tubes, and shipped overnight in insulated thermal containers packed with frozen icepacks to the processing laboratory at Loma Linda, CA. Buffy coat was removed and diluted to a final volume of 8 mL with phosphate buffered saline, then aliquoted into straws and frozen at −180 °C in nitrogen vapor.
2.5. DNA Isolation and DNA Methylation Analysis
Genomic DNA from buffy coat samples was isolated using the Quick-gDNA MiniPrep kit (Zymo Research, Irvine, CA, USA) according to the manufacturer’s protocol. Genome-wide methylation analysis was carried out using the Illumina Infinium HumanMethylation450 BeadChip platform at the UCLA Neuroscience Genomics Core in two separate batches. Raw data was summarized into BeadStudio IDAT files for further analysis.
2.6. Methylation Data Analysis
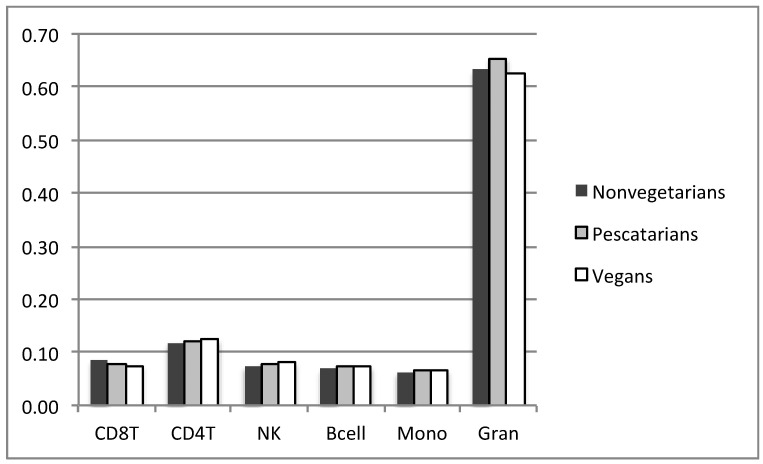
Methylation data, based on methylation beta values, were analyzed using the Partek Genomic Suite (Partek, St. Lois, MO, USA). Comparative analyses were carried out separately between vegans and nonvegetarians and between vegans and pescatarians. Batch correction was carried out using the ANOVA-based batch correction method embedded within the commercial program Partek. We also used the R minfi package, based on Houseman’s method to estimate blood cell heterogeneity in buffy coat samples [15], with the analysis indicating no significant variation between cell types between the three groups as shown on Figure 1.
Figure 1.
Composition of cell types in blood samples estimated by Houseman method using R minfi package.
Raw data was normalized using the SWAN (Subset-quantile With Array Normalization) method. Normalized data were subjected to quality control analysis, and nine samples with distorted signal frequency histograms were culled out. Primary normalization was followed by batch effect correction and quantile normalization. Data from probes representing CpG sites from chromosomes X and Y and those close to known SNPs (±10 nucleotides) were removed from further analysis. Data from the remaining probes were analyzed to identify sites displaying differential CpG methylation between the three diets. Initial filtering using an FDR < 0.05 cutoff for nonvegetarians revealed only one site, consistent with the expected modest differences due to diet. This CpG site was also the only one to appear with FDR cutoffs of 0.1, 0.2, and 0.3. Subsequently, relaxed conditions of an ANOVA procedure with a Fold Change cutoff = 1.2 (20% difference) and a p-value cutoff = 0.05 were employed to detect differentially methylated sites. Differentially methylated genes identified by this explorative approach, which does not correct for multiple testing, were then subjected to pathway analysis.
2.7. Biological Pathway Analyses
To analyze in more detail the potential biological effects of a vegan diet due to variations in methylation patterns, we conducted biological pathway analyses using the Ingenuity Pathway Analysis (IPA) web-based analysis tool, as well as DAVID Bioinformatics Resources [16]. These analyses allowed us to statistically evaluate if the CpG differentially methylated sites we identified were specifically enriched with genes that are engaged in certain pathways, as well as any possible crosstalk between them. The original file generated by analysis in the Partek suite, containing the name of each gene, fold change, and P value information was uploaded into the IPA system. Using a cut-off value of 1.25 for fold change, a new dataset was produced, which was used for Core Analysis to identify relevant canonical pathways. Pathways with P values (Fisher’s test) of less than 0.05 were considered significant. The same list of genes was analyzed with DAVID tools to identify enriched functional-related gene groups.
3. Results
3.1. Characterization of Study Subjects
We conducted three group comparisons using the Student’s t--test or the Fisher’s exact test as appropriate: Vegan vs. nonvegetarian, vegan vs. pescatarian, and pescatarian vs. nonvegetarian. Comparisons of selected characteristics of the study sample are shown in Table 1.
Table 1.
Selected characteristics of study sample comparing vegans, pesco vegetarians, and omnivores.
| Demographic and Lifestyle | n = 38 | n = 41 | n = 68 | Vegan vs. Omni | Pesco vs. Omni | Vegan vs. Pesco | |||
|---|---|---|---|---|---|---|---|---|---|
| Males (%) | |||||||||
| Age at collection, mean ± SD (years) | 52.63 | 48.78 | 47.06 | 0.58 | 0.86 | 0.73 | |||
| BMI at collection, mean ± SD | 67.78 (8.50) | 69.93 (7.87) | 66.42 (6.73) | 0.37 | 0.02 | 0.25 | |||
| Exercise, mean ± SD (min/week) | 23.62 (4.23) | 25.36 (3.63) | 27.07 (3.69) | <0.0001 | 0.02 | 0.05 | |||
| Sleep hours, mean ± SD (hrs/day) | 152.93 (109.59) | 76.68 (86.64) | 69.23 (95.72) | 0.0001 | 0.58 | 0.002 | |||
| Perception of one’s own health (%) | 7.30 (0.81) | 7.03 (0.95) | 6.94 (0.93) | 0.05 | 0.65 | 0.18 | |||
| Excellent | 0.07 | 0.82 | 0.22 | ||||||
| Good | 25.71 | 22.50 | 17.65 | ||||||
| Fair | 54.29 | 70.00 | 75.00 | ||||||
| Prevalent cancer (%) | 20.00 | 7.50 | 7.35 | ||||||
| Yes | 0.57 | 0.66 | 0.91 | ||||||
| No | 10.53 | 9.76 | 7.35 | ||||||
| CVD comorbidities (%) | 89.47 | 90.24 | 92.65 | ||||||
| None | 0.35 | 0.74 | 0.57 | ||||||
| 1 or more | 76.32 | 70.73 | 67.65 | ||||||
| Cigarette smoke (%) | 23.68 | 29.27 | 32.35 | ||||||
| Never | 0.05 | 0.51 | 0.02 | ||||||
| Ever | 97.37 | 80.49 | 85.29 | ||||||
| 2.63 | 19.51 | 14.71 | |||||||
| Dietary intake, mean ± SD | |||||||||
| Energy (kcal/d) | |||||||||
| Carbohydrate (% of kcal) | 1769.82 (585.13) | 2029.3 (609.7) | 1927.77 (667.22) | 0.23 | 0.43 | 0.06 | |||
| Fat (% of kcal) | 57.58 (6.96) | 52.83 (7.17) | 49.74 (8.13) | <0.0001 | 0.05 | 0.004 | |||
| Protein (% of kcal) | 28.72 (6.92) | 33.20 (7.29) | 35.66 (7.85) | <0.0001 | 0.11 | 0.007 | |||
| Vegetable protein (% of protein) | 13.70 (2.80) | 13.97 (2.42) | 14.61 (2.21) | 0.071 | 0.07 | 0.65 | |||
| Animal protein (% of protein) | 96.87 (2.57) | 80.39 (12.56) | 60.63 (14.82) | <0.0001 | <0.0001 | <0.0001 | |||
| Essential amino acids | 3.89 (2.33) | 19.92 (12.41) | 39.41 (14.75) | <0.0001 | <0.0001 | <0.0001 | |||
| Leucine (% of protein) | |||||||||
| Valine (% of protein) | 6.98 (0.48) | 7.40 (0.35) | 7.61 (0.36) | <0.0001 | 0.003 | <0.0001 | |||
| Isoleucine (% of protein) | 4.66 (0.25) | 4.89 (0.21) | 4.98 (0.21) | <0.0001 | 0.03 | <0.0001 | |||
| Histidine (% of protein) | 3.95 (0.24) | 4.12 (0.20) | 4.29 (0.19) | <0.0001 | <0.0001 | 0.0007 | |||
| Methionine (% of protein) | 2.42 (0.13) | 2.45 (0.08) | 2.54 (0.1) | <0.0001 | <0.0001 | 0.24 | |||
| Tryptophan (% of protein) | 1.50 (0.12) | 1.73 (0.17) | 1.92 (0.16) | <0.0001 | <0.0001 | <0.0001 | |||
| Threonine (% of protein) | 1.26 (0.07) | 1.23 (0.06) | 1.22 (0.06) | 0.002 | 0.16 | 0.1 | |||
| Lysine (% of protein) | 3.5 (0.26) | 3.47 (0.20) | 3.55 (0.17) | 0.33 | 0.04 | 0.54 | |||
| Phenylalanine (% of protein) | 4.47 (0.48) | 4.93 (0.48) | 5.55 (0.64) | <0.0001 | <0.0001 | <0.0001 | |||
| Non-essential amino acids | 4.88 (0.24) | 4.88 (0.11) | 4.75 (0.16) | 0.005 | <0.0001 | 0.99 | |||
| Glutamate (% of protein) | |||||||||
| Aspartate (% of protein) | 23.07 (1.94) | 22.82 (1.58) | 22.18 (1.64) | 0.010 | 0.05 | 0.53 | |||
| Alanine (% of protein) | 10.20 (1.33) | 9.50 (0.79) | 9.07 (0.71) | <0.0001 | 0.004 | 0.005 | |||
| Cysteine (% of protein) | 4.40 (0.38) | 4.25 (0.27) | 4.29 (0.23) | 0.06 | 0.46 | 0.05 | |||
| Arginine (% of protein) | 1.61 (0.14) | 1.65 (0.16) | 1.65 (0.16) | 0.21 | 0.95 | 0.28 | |||
| Tyrosine (% of protein) | 6.79 (0.79) | 6.12 (0.73) | 5.61 (0.66) | <0.0001 | 0.0003 | 0.0002 | |||
| Glycine (% of protein) | 3.04 (0.28) | 3.24 (0.25) | 3.38 (0.20) | <0.0001 | 0.002 | 0.001 | |||
| Proline (% of protein) | 4.27 (0.31) | 3.92 (0.34) | 3.79 (0.32) | <0.0001 | 0.05 | <0.0001 | |||
| Serine (% of protein) | 6.65 (0.92) | 7.24 (0.86) | 7.31 (0.73) | <0.0001 | 0.64 | 0.004 | |||
| Micronutrients | 4.87 (0.29) | 4.95 (0.16) | 4.79 (0.19) | 0.10 | <0.0001 | 0.12 | |||
| Vitamin B2, mg/1000 kcal | |||||||||
| Vitamin B6, mg/1000 kcal | 2.85 (3.28) | 3.64 (5.18) | 2.78 (6.62) | 0.95 | 0.43 | 0.42 | |||
| Vitamin B-12, mcg/1000 kcal | 3.56 (7.04) | 8.54 (15.42) | 5.14 (10.21) | 0.4 | 0.17 | 0.07 | |||
| Folate, mcg/1000 kcal | 3.93 (4.43) | 7.64 (9.38) | 6.19 (11.23) | 0.24 | 0.49 | 0.03 | |||
| Choline, mg/1000 kcal | 460.0 (387.8) | 440.1 (193.0) | 401.9 (214.1) | 0.19 | 0.35 | 0.67 | |||
| Betaine, mg/1000 kcal | 117.0 (21.73) | 122.9 (29.40) | 136.9 (27.61) | 0.0002 | 0.01 | 0.32 | |||
| 0.91 | 0.6 |
Compared to vegans, BMI was higher among pescatarians (p = 0.05) and nonvegetarians (p < 0.0001), and pescatarians had lower BMI than nonvegetarians (p = 0.02). Exercise duration (min/week) was lower among pescatarians (p = 0.002) and nonvegetarians (p = 0.0001) compared to vegans, but no significant difference was observed between pescatarians and nonvegetarians. Prevalence of cancer, CVD comorbidities, and health perception were not significantly different among the comparison groups. Adventists notably are a non-smoking population, and in this sample < 3% of vegans, 20% pescatarians, and <15% of non-vegetarians ever smoked. Compared to vegans (67.8 y), average age was not significantly different among pescatarians (69.9 y) or nonvegetarians (66.4 y). However, pescatarians were older than nonvegetarians (p = 0.02) (Table 1).
Table 1 also shows dietary intake characteristics of our study subjects. Compared to vegans, carbohydrate intake was lower among nonvegetarians (p < 0.0001) and pescatarians (p = 0.004), and fat intake was higher among nonvegetarians (p < 0.0001) and pescatarians (p = 0.007). Though energy and total protein intake (as % of energy) did not differ between the comparison groups, we found significant trends in the intake of vegetable protein and animal protein (as % of protein). Not surprisingly, vegetable protein was highest among vegans, intermediate among pescatarians and lowest among nonvegetarians, and animal protein intake was highest among nonvegetarians, followed by pescatarians and negligible amounts among vegans (p < 0.0001 for all comparison groups). Among the essential amino acids (where the source must come from the diet), intake tended to be highest among nonvegetarians, intermediate among pescatarians, and lowest among vegans (p-values ≤ 0.05 for nearly all comparison groups), with the exceptions of tryptophan, threonine, and phenylalanine. Among the non-essential amino acids, we found similar trends for the intake of tyrosine and proline; however, intake of glutamate, aspartate, arginine, glycine, and serine tended to be highest among vegans, then pescatarians, and lowest among nonvegetarians (p-values ≤ 0.05 for nearly all comparison groups). Among the nutrients involved in one-carbon metabolism, dietary intake of vitamin B2, vitamin B6, folate, and betaine were not significantly different between the comparison groups. Vitamin B12 was significantly higher in pescatarians compared to vegans (p = 0.03), and compared to nonvegetarians, choline intake was lower (p = 0.0002) among vegans and pescatarians (p = 0.01).
3.2. Identification of Differential Methylation Sites
Methylation data was obtained and analyzed using the Infinium HumanMethylation450 beadchip array and Partek Genomic Suite software. Initial comparison of methylation profiles between vegans and nonvegetarians using the following statistical parameters: FDR (False Discovery Rate) < 0.05 and fold change difference more than 1.2 or less than −1.2, showed that no CpG sites passed these criteria. The one site that did meet the FDR criteria (within the OR1M1 olfactory receptor gene) did not meet the fold change criteria, and was therefore excluded from further analysis. For the comparison between vegans and pescatarians, no sites met the FDR criteria.
We then used relaxed statistical conditions by using unadjusted p-values < 0.05 and a Fold-Change (FC) cutoff of 1.2, we found 523 CpG sites that passed these criteria when we compared vegan vs. nonvegetarians, of which 424 were located within gene areas (Table S1). We then performed the same analysis to compare methylation data between the same vegan group and the pescatarians. This led to the detection of 358 CpG sites, of which 271 were located within known gene areas (Table S2). A comparison of the differentially methylated CpG sites between nonvegetarians and pescatarians revealed that 77 sites are common for both these diets, and that 55 of them are within known genes (Table S3).
To further explore whether the inclusion of animal-derived proteins in the diet may be reflected in epigenetic differences, we compared the lists of the ten top genes displaying differentially methylated CpG sites that showed the highest differences in methylation status for nonvegetarians and pescatarians (Table 2 and Table 3, correspondingly).
Table 2.
Ten top genes wittabh the greatest differential CpG methylation status in vegans vs. nonvegetarians.
| Entrez Gene Name | Symbol | Fold Change | Location | Type(s) |
|---|---|---|---|---|
| mitochondrial ribosomal protein L19 | MRPL19 | 2.517 | Cytoplasm | other |
| proline rich 7, synaptic | PRR7 | 2.071 | Other | other |
| glutathione S-transferase C-terminal domain containing | GSTCD | 1.879 | Cytoplasm | enzyme |
| chromosome 7 open reading frame 50 | C7orf50 | 1.743 | Other | other |
| dynein axonemal heavy chain 10 | DNAH10 | 1.636 | Cytoplasm | other |
| solute carrier family 38 member 6 | SLC38A6 | 1.619 | Plasma Membrane | transporter |
| glutathione S-transferase theta 1 | GSTT1 | 1.594 | Cytoplasm | enzyme |
| calcium voltage-gated channel auxiliary subunit beta 2 | CACNB2 | 1.541 | Plasma Membrane | ion channel |
| family with sequence similarity 19 member A5, C-C motif chemokine like | FAM19A5 | 1.524 | Extracellular Space | other |
| transmembrane protein 229A | TMEM229A | 1.523 | Other | other |
Genes highlighted in grey are common for both nonvegetarians and pescatarians vs. vegans comparisons.
Table 3.
Ten top genes with the greatest differential CpG methylation status in vegans vs. pescatarians.
| Entrez Gene Name | Symbol | Fold Change | Location | Type(s) |
|---|---|---|---|---|
| purine rich element binding protein G | PURG | 2.488 | Other | other |
| proline rich 7, synaptic | PRR7 | 2.421 | Plasma Membrane | other |
| mitochondrial ribosomal protein L19 | MRPL19 | 2.067 | Cytoplasm | other |
| catalase | CAT | 1.864 | Cytoplasm | enzyme |
| kinesin family member 15 | KIF15 | 1.523 | Nucleus | other |
| solute carrier family 38 member 6 | SLC38A6 | 1.507 | Plasma Membrane | transporter |
| proteasome 26S subunit, non-ATPase 5 | PSMD5 | 1.478 | Other | other |
| Rap associating with DIL domain | RADIL | 1.433 | Cytoplasm | other |
| amyloid beta precursor protein binding family B member 2 | APBB2 | 1.431 | Cytoplasm | other |
Genes highlighted in grey are common for both nonvegetarians and pescatarians vs. vegans comparisons.
Three genes that appeared in the results from both analyses are highlighted in Table 2 and Table 3, indicating their highly methylated status in vegans as compared to both pescatarians and nonvegetarians.
A gene list created by comparing the differentially methylated CpG sites observed in nonvegetarians and vegans was used to perform DAVID functional annotation clustering using its own Knowledgebase. This analysis revealed that out of 49 identified functional groups of genes (Table S4), the top cluster, as shown in Table 4, demonstrates enrichment of homeobox transcriptional factors. This enrichment is significant by a variety of statistical tests. The potential of these transcription factors to amplify differential activation may indicate that the presence or absence of animal proteins in the diet may lead to much larger differences in gene expression regulation than the relatively modest fold-changes detected by an epigenetics approach might suggest.
Table 4.
Functional annotation cluster 1 report for vegans vs. nonvegetarians run with high classification stringency.
| Annotation Cluster 1 | Enrichment Score: 5.206834531318599 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Term | Count | % | p Value | List Total | Fold Enrichment | Bonferroni | Benjamini | FDR |
| INTERPRO | IPR017970:Homeobox, conserved site | 16 | 4.98 | 4.5 × 10−7 | 299 | 5.23 | 2.9 × 10−4 | 2.9 × 10−4 | 6.7 × 10−4 |
| UP_KEYWORDS | Homeobox | 17 | 5.30 | 3.2 × 10−6 | 317 | 4.21 | 9.5 × 10−4 | 4.7 × 10−4 | 0.004 |
| INTERPRO | IPR001356:Homeodomain | 17 | 5.30 | 4.1 × 10−6 | 299 | 4.12 | 0.003 | 0.001 | 0.006 |
| UP_SEQ_FEATURE | DNA-binding region:Homeobox | 14 | 4.36 | 9.7 × 10−6 | 313 | 4.70 | 0.011 | 0.011 | 0.016 |
| SMART | SM00389:HOX | 17 | 5.30 | 2.9 × 10−6 | 197 | 3.47 | 0.005 | 0.005 | 0.036 |
| INTERPRO | IPR009057:Homeodomain-like | 18 | 5.61 | 3.3 × 10−5 | 299 | 3.33 | 0.022 | 0.007 | 0.049 |
Term: Biological process. Count: Number of genes involved in this pathway. %: percentage of involved genes to the total number of genes in this analysis.
Similar results were obtained when we performed the same analysis under the same conditions using the gene list based on differential methylation detected in pescatarians vs. vegans. Table 5 shows that homeobox transcription factors again form the top functional cluster among all the genes used in analysis. However, statistical characteristics for pescatarians are weaker (Table 5).
Table 5.
Functional annotation cluster 1 report run for vegans vs. pescatarians run with high classification stringency.
| Annotation Cluster 1 | Enrichment Score: 1.945388640497003 | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Category | Term | Count | % | p Value | List Total | Fold Enrichment | Bonferroni | Benjamini | FDR |
| UP_SEQ_FEATURE | DNA-binding region:Homeobox | 8 | 3.76 | 0.0033 | 203 | 4.14 | 0.88 | 0.88 | 4.79 |
| INTERPRO | IPR020479:Homeodomain, metazoa | 6 | 2.82 | 0.0035 | 205 | 5.90 | 0.81 | 0.81 | 4.85 |
| INTERPRO | IPR017970:Homeobox, conserved site | 8 | 3.76 | 0.0051 | 205 | 3.81 | 0.91 | 0.71 | 7.05 |
| UP_KEYWORDS | Homeobox | 8 | 3.76 | 0.0171 | 208 | 3.02 | 0.99 | 0.52 | 20.09 |
| INTERPRO | IPR001356:Homeodomain | 8 | 3.76 | 0.0230 | 205 | 2.83 | 1.00 | 0.89 | 28.36 |
| SMART | SM00389:HOX | 8 | 3.76 | 0.0323 | 123 | 2.62 | 0.98 | 0.86 | 31.44 |
| INTERPRO | IPR009057:Homeodomain-like | 9 | 4.23 | 0.0327 | 205 | 2.42 | 1.00 | 0.90 | 37.89 |
It is important to note that, out of 8 genes encoding homeobox transcription factors detected in pescatarians, 3 are found also in nonvegetarians, which indicate on nonrandom differences in methylation patterns (Table 6).
Table 6.
CpG sites identified as differentially methylated in transcription factor genes in pescatarians.
| ID | Gene Name | FC in Omnivores | FC in Piscvores |
|---|---|---|---|
| BARHL1 | BarH like homeobox 1(BARHL1) | −1.23555 | |
| ISL1 | ISL LIM homeobox 1(ISL1) | 1.23352 | 1.2188 |
| NKX3-2 | NK3 homeobox 2(NKX3-2) | 1.25585 | 1.26999 |
| NKX6-1 | NK6 homeobox 1(NKX6-1) | −1.32335 | |
| EVX1 | even-skipped homeobox 1(EVX1) | 1.27498 | |
| HOXA2 | homeobox A2(HOXA2) | 1.24374 | |
| HOXC9 | homeobox C9(HOXC9) | 1.29757 | |
| RAX | retina and anterior neural fold homeobox(RAX) | 1.27834 | 1.24775 |
Genes that are also differentially methylated in nonvegetarians are highlighted in grey.
3.3. Pathway Analysis
To analyze in more detail the potential biological effects of a vegan diet due to variations in methylation patterns, we employed the DAVID (Database for Annotation, Visualization and Integrated Discovery) online resource [16]. This resource uses several pathway databases, including the KEGG (Kyoto Encyclopedia of Genes and Genomes) database, which contains pathway maps. Such analyses are useful in evaluating the biological relevance of high-throughput data, particularly when relaxed statistics have been used. In particular, pathway analysis allowed us to statistically evaluate whether the group of identified genes is specifically enriched with genes that are engaged in certain pathways/processes.
The pathway analysis of pescatarians vs. vegans found only 3 processes that were enriched in this group of genes (Table 7). However, two of these processes were also found in the vegan vs. nonvegetarian analysis (Table 8): The Hippo signaling pathway and the Glutamatergic Synapse pathway. These two pathways are highlighted in table for pescatarians (Table 7).
Table 7.
(Kyoto Encyclopedia of Genes and Genomes) Pathways enriched in genes with differential methylation in pescatarians.
| Term | Count | % | p Value | Fold Enrichment | Bonferroni | Benjamini | FDR |
|---|---|---|---|---|---|---|---|
| hsa04390:Hippo signaling pathway | 7 | 3.29 | 0.0031 | 4.76 | 0.34 | 0.34 | 3.58 |
| hsa05014:Amyotrophic lateral sclerosis (ALS) | 4 | 1.88 | 0.0120 | 8.21 | 0.80 | 0.55 | 13.16 |
| hsa04724:Glutamatergic synapse | 5 | 2.35 | 0.0235 | 4.50 | 0.96 | 0.65 | 24.25 |
Term: Name of KEGG pathway; Count: Number of genes involved in this pathway; %: percentage of involved genes to the total number of genes in this analysis.Pathways that were also found in pathway analysis of nonvegetarians are highlighted in grey.
Table 8.
KEGG Pathways enriched in genes with differential methylation in nonvegetarians.
| Term | Count | % | p Value | Fold Enrichment | Bonferroni | Benjamini | FDR |
|---|---|---|---|---|---|---|---|
| hsa05033:Nicotine addiction | 5 | 1.56 | 0.0032 | 8.04 | 0.42 | 0.42 | 3.82 |
| hsa04723:Retrograde endocannabinoid signaling | 6 | 1.87 | 0.0195 | 3.82 | 0.97 | 0.82 | 21.38 |
| hsa04921:Oxytocin signaling pathway | 7 | 2.18 | 0.0279 | 3.00 | 0.99 | 0.80 | 29.23 |
| hsa04390:Hippo signaling pathway | 7 | 2.18 | 0.0287 | 2.98 | 0.99 | 0.71 | 29.94 |
| hsa04727:GABAergic synapse | 5 | 1.56 | 0.0417 | 3.78 | 1.00 | 0.77 | 40.59 |
| hsa04810:Regulation of actin cytoskeleton | 8 | 2.49 | 0.0429 | 2.45 | 1.00 | 0.72 | 41.46 |
Term: Name of KEGG pathway; Count: Number of genes involved in this pathway; %: percentage of involved genes to the total number of genes in this analysis.Pathways that include proteins involved in GABA and Glutamate metabolism are highlighted in grey.
Table 8 shows 6 top pathways detected among the genes with differential methylation noted in nonvegetarians. Although only 2 of these pathways are the same as those noted for pescatarians, some of the pathways share overlapping sets of genes and this can lead to some redundancy. For example, the four pathways marked in grey in Table 8 share genes involved in processes linked to GABA regulation and thus form one cluster.
4. Discussion
We performed comparative DNA methylation analyses of DNA isolated from the blood cells of vegans versus nonvegetarians and of vegans versus pescatarians using the Illumina Infinium 450k human assay. Importantly, in this study, DNA methylation analyses of vegans against individuals with similar but not identical diets containing animal-derived sources of protein led to the identification of genes clustered in similar but not completely identical functional groups.
Identification and interpretation of DNA methylation differences associated with diet preferences in humans are complicated by several interdependent aspects. The fact that humans are the study subjects brings significant biological variability to genotype-environment (GxE) interactions, leading to inter- and intra-individual (i.e., over time) methylome variations in humans [17]. Another factor relevant to this study is the likelihood that vegans in general tend to be more aware of the need for a healthy lifestyle, leading to differences in BMI and physical activity between our two subgroups. These differences in BMI and physical activity have the potential to contribute to our observed differences in methylation patterns. Unfortunately, it is not possible currently to dissect out the differential impacts from these factors due to the limited numbers of subjects in this study. Therefore, it may be appropriate to interpret our findings in the context of the broader emphasis on a healthy lifestyle exhibited by vegans.
We also note that there are multiple mechanisms through which protein expression and metabolism—and thereby, health outcomes—can be modulated, of which epigenetic changes are only one. Modest changes in gene expression have been shown in studies of Prudent and Western dietary patterns in healthy individuals [18]. Therefore, we expected to find that any epigenetic changes related to diet preferences, especially in the DNA of blood cells, should also be modest, if indeed, they could be detected. As noted above, pathway analysis statistically evaluates all observed changes in activation or inactivation of proteins engaged in a particular pathway. The fact that pathway analysis did detect differences in the modulation of specific pathways, even though the only input was changes in methylation status, is noteworthy.
All of these factors contribute to the modest ability of these types of analyses to detect the full extent of gene expression changes that could be related to diet. To address these challenges and to increase the likelihood of detecting genuine associations of methylome changes with diet preferences, relaxed criteria were applied. These relaxed criteria increase the likelihood of detecting of both true and false positives. A rough estimation regarding the relative numbers of true and false positives can be suggested from analysis of the top ten most differentially methylated genes (Table 2 and Table 3), where 3 genes, or approximately 30% of this top grouping, were identified in both analyses. Another independent layer of analysis designed to increase the probability of detecting true differences was to search for identical sites (genes) found to be differentially methylated in DNA from individuals who follow either of two closely related but not identical diets, where these genes are involved in well-known biological processes/pathways. That is, identification of changes in the methylation of the same genes and pathways in individuals who consume no animal proteins as compared to those who restrict their diet to only fish, and as compared to those who are to full omnivores, strengthens the conclusions we can draw.
To our knowledge, this is the first large-scale attempt to apply an epigenome-wide approach to examine the relationship between diet—and in particular, a diet restricted to plant-based food-and methylome changes. In general, the magnitude of individual changes was relatively modest—we used a cut-off of 1.2 for the Partek analysis. Such changes are in line with what has been previously reported regarding the relationship between methylation levels and nutrition. For example, fortification of food with the methyl donor, folic acid, increased the methylation level of a DM site within the IGF2 gene in human blood cells by 4.5%, a value that is believed to be comparable to results obtained in other mammals [19].
Genome-wide methylation analysis of 147 individuals, 38 of which are vegans, allowed us to obtain sufficient data to gain biologically interesting results for epigenetic differences in methylation in vegans versus pescatarians and in vegans versus nonvegetarians. Interestingly, we found that vegans had a higher methylation status in the majority of the differentially methylated sites, with DNA hypomethylation occurring in only 4% of all DM probes in the nonvegetarian comparison; this value was 33% for the pescatarian comparison. This is consistent with a study comparing methylation status between two empirically-defined dietary patterns, where a prudent diet (characterized by high intakes of fruits and vegetables) compared to a Western diet (characterized by high intake of grains, potatoes, meats and oils) was associated with less DNA hypomethylation [20].
One of the most intriguing outcome of our analysis was the simultaneously increased methylation of regulatory elements of genes involved in transport of two major neurotransmitters: GABA (inhibitory), and glutamate (excitatory) in nonvegetarians. Interestingly, microbiome work by David et al. also found altered expression of genes involved in amino acid metabolism (GABA and glutamate) and distinct enrichment patterns associated with animal- and plant-based diets [21]. This finding may be partially explained by the amino acid composition of the vegan diet, as a number of important products are synthesized from amino acids.
Our finding that transcriptional factors, and in particular, homeobox regulators, were enriched in the list of differentially methylated genes (Table 4, Table 5 and Table 6), raises the question of how many genes may have been affected by these diet restrictions. This is also true for the HIPPO pathway, where differential methylation of several genes, as well as pathway enrichment, were found in both non-vegan diets. HIPPO pathway plays an important role in the regulation of tissue homeostasis [22]. Direct investigation regarding the expression of these genes would be quite useful in efforts to further understand the potential role of diet in health and disease development.
5. Conclusions
We performed genome-wide comparisons of methylation patterns in the DNA of blood cells isolated from vegans, pescatarians and nonvegetarians. The results allowed us to identify CpG sites that may display differential DNA methylation. We also found that the set of genes with differentially methylated CpG sites in vegans are enriched in specific common functional clusters/pathways, when compared with DNA isolated from either pescatarians or nonvegetarians. This suggests that these epigenetic changes are diet-related and have the potential to control specific physiological processes. Future efforts focused on direct investigation of gene expression will continue to expand our understanding of the influence of nutrition on major biological processes that regulate health and quality of life.
Acknowledgments
The authors thank Gary Fraser and Michael Orlich (Loma Linda University, School of Public Health) for discussions and comments that greatly improved the manuscript.
Supplementary Materials
The following are available online at https://www.mdpi.com/2075-4655/4/4/28/s1, Table S1. GpC sites found to be differentially methylated in vegans (VE) compared to nonvegetarians/omnivores (OV) using a fold change cut off ≥ 1.2 and a p value ≤ 0.05. This Excel file (.xls) was generated using Partek Genomic Suite software (Partek, St Lois, MO, USA). Table S2. GpC sites found to be differentially methylated in vegans (VE) compared to pescatarians (PV) using a fold change cut off ≥ 1.2 and a p value ≤ 0.05. This Excel file (.xls) was generated using Partek Genomic Suite software (Partek, St Lois, MO, USA). Table S3. List of genes in which CpG sites are differentially methylated in vegans compared to omnivores and pescatarians. Table 4. List of 49 functional clusters generated by analysis of omnivore-specific genes by the DAVID online resource.
Author Contributions
Conception and Design: V.F. and P.J.D.-H.; Technical support: V.F. and V.L.; Analysis and Interpretation of Data: V.F., A.E., and K.J.-S.; Drafting the Article: V.F., K.J.-S., and P.J.D.-H.; Revising of the Article for intellectual content: V.F., A.E., K.J.-S., X.C., C.W., and P.J.D.-H. All authors have read and agreed to the published version of the manuscript.
Funding
Supported in part by NIH grant 5RO1 CA 094594 for the collection of samples, as well as by internal funding through Loma Linda University (GRASP grant 2120211) for DNA methylation analysis. In addition, the Adventist Health Study-2 is funded in part by the Ardmore Institute of Health.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Craig W.J., Mangels A.R. American Dietetic A: Position of the American Dietetic Association: Vegetarian diets. J. Am. Diet Assoc. 2009;109:1266–1282. doi: 10.1016/j.jada.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 2.Dinu M., Abbate R., Gensini G.F., Casini A., Sofi F. Vegetarian, vegan diets and multiple health outcomes: A systematic review with meta-analysis of observational studies. Crit. Rev. Food Sci. Nutr. 2017;57:3640–3649. doi: 10.1080/10408398.2016.1138447. [DOI] [PubMed] [Google Scholar]
- 3.U.S. DoHaHSaUSDoA: 2015–2020 Dietary Guidelines for Americans, 8th ed. [(accessed on 8 December 2020)];2015 Available online: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines/guidelines/
- 4.Mutch D.M., Wahli W., Williamson G. Nutrigenomics and nutrigenetics: The emerging faces of nutrition. FASEB J. 2005;19:1602–1616. doi: 10.1096/fj.05-3911rev. [DOI] [PubMed] [Google Scholar]
- 5.Sapienza C., Issa J.P. Diet, Nutrition, and Cancer Epigenetics. Annu. Rev. Nutr. 2016;36:665–681. doi: 10.1146/annurev-nutr-121415-112634. [DOI] [PubMed] [Google Scholar]
- 6.Schaible T.D., Harris R.A., Dowd S.E., Smith C.W., Kellermayer R. Maternal methyl-donor supplementation induces prolonged murine offspring colitis susceptibility in association with mucosal epigenetic and microbiomic changes. Hum. Mol. Genet. 2011;20:1687–1696. doi: 10.1093/hmg/ddr044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen P.Y., Ganguly A., Rubbi L., Orozco L.D., Morselli M., Ashraf D., Jaroszewicz A., Feng S., Jacobsen S.E., Nakano A. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol. Genom. 2013;45:565–576. doi: 10.1152/physiolgenomics.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Strakovsky R.S., Zhang X., Zhou D., Pan Y.X. Gestational high fat diet programs hepatic phosphoenolpyruvate carboxykinase gene expression and histone modification in neonatal offspring rats. Pt 11J. Physiol. 2011;589:2707–2717. doi: 10.1113/jphysiol.2010.203950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Saldanha S.N., Tollefsbol T.O. Impact of epigenetic dietary compounds on transgenerational prevention of human diseases. AAPS J. 2014;16:27–36. doi: 10.1208/s12248-013-9538-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tobi E.W., Lumey L.H., Talens R.P., Kremer D., Putter H., Stein A.D., Slagboom P.E., Heijmans B.T. DNA methylation differences after exposure to prenatal famine are common and timing- and sex-specific. Hum. Mol. Genet. 2009;18:4046–4053. doi: 10.1093/hmg/ddp353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Butler T.L., Fraser G.E., Beeson W.L., Knutsen S.F., Herring R.P., Chan J., Sabate J., Montgomery S., Haddad E., Preston-Martin S., et al. Cohort profile: The Adventist Health Study-2 (AHS-2) Int. J. Epidemiol. 2008;37:260–265. doi: 10.1093/ije/dym165. [DOI] [PubMed] [Google Scholar]
- 12.Jaceldo-Siegl K., Knutsen S.F., Sabate J., Beeson W.L., Chan J., Herring R.P., Butler T.L., Haddad E., Bennett H., Montgomery S., et al. Validation of nutrient intake using an FFQ and repeated 24 h recalls in black and white subjects of the Adventist Health Study-2 (AHS-2) Public Health Nutr. 2010;13:812–819. doi: 10.1017/S1368980009992072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Willett W. Nutritional Epidemiology. 2nd ed. Oxford University Press; New York, NY, USA: 1998. [Google Scholar]
- 14.Rizzo N.S., Jaceldo-Siegl K., Sabate J., Fraser G.E. Nutrient profiles of vegetarian and nonvegetarian dietary patterns. J. Acad. Nutr. Diet. 2013;113:1610–1619. doi: 10.1016/j.jand.2013.06.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Aryee M.J., Jaffe A.E., Corrada-Bravo H., Ladd-Acosta C., Feinberg A.P., Hansen K.D., Irizarry R.A. Minfi: A flexible and comprehensive Bioconductor package for the analysis of Infinium DNA methylation microarrays. Bioinformatics. 2014;30:1363–1369. doi: 10.1093/bioinformatics/btu049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huang da W., Sherman B.T., Lempicki R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bock C., Walter J., Paulsen M., Lengauer T. Inter-individual variation of DNA methylation and its implications for large-scale epigenome mapping. Nucleic Acids Res. 2008;36:e55. doi: 10.1093/nar/gkn122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bouchard-Mercier A., Paradis A.M., Rudkowska I., Lemieux S., Couture P., Vohl M.C. Associations between dietary patterns and gene expression profiles of healthy men and women: A cross-sectional study. Nutr. J. 2013;12:24. doi: 10.1186/1475-2891-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steegers-Theunissen R.P., Obermann-Borst S.A., Kremer D., Lindemans J., Siebel C., Steegers E.A., Slagboom P.E., Heijmans B.T. Periconceptional maternal folic acid use of 400 microg per day is related to increased methylation of the IGF2 gene in the very young child. PLoS ONE. 2009;4:e7845. doi: 10.1371/journal.pone.0007845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang F.F., Morabia A., Carroll J., Gonzalez K., Fulda K., Kaur M., Vishwanatha J.K., Santella R.M., Cardarelli R. Dietary patterns are associated with levels of global genomic DNA methylation in a cancer-free population. J. Nutr. 2011;141:1165–1171. doi: 10.3945/jn.110.134536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.David L.A., Maurice C.F., Carmody R.N., Gootenberg D.B., Button J.E., Wolfe B.E., Ling A.V., Devlin A.S., Varma Y., Fischbach M.A., et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–563. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pan D. The hippo signaling pathway in development and cancer. Dev. Cell. 2010;19:491–505. doi: 10.1016/j.devcel.2010.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.