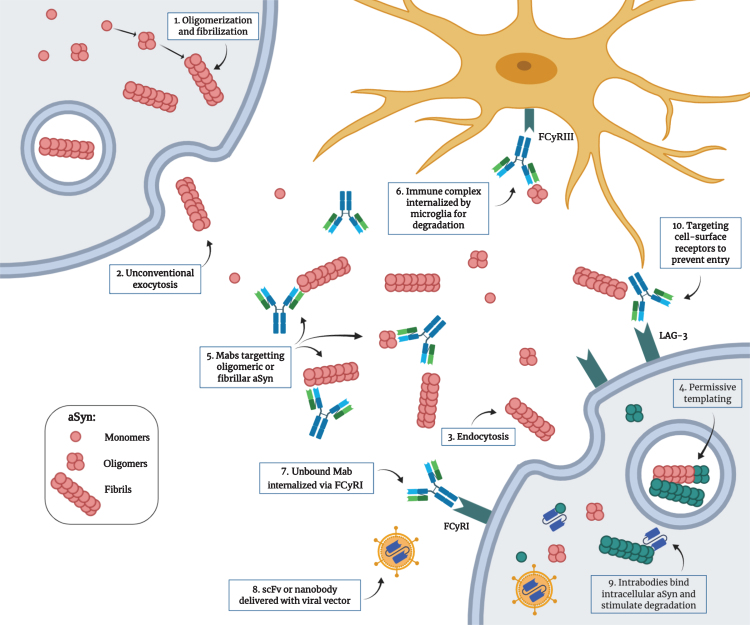
Fig.2.
Proposed mechanism for the pathological transmission of aSyn and possible immunotherapeutic targets. 1) Post-translational modifications, cellular stress and mutations can trigger the misfolding and aggregation of aSyn into oligomers and fibrils. 2) Toxic forms of aSyn can be released in the extracellular space by unconventional exocytosis. 3) Once in the extracellular space, aggregated aSyn can be internalized by neighboring cells by endocytosis through binding of specific receptors. 4) Misfolded aSyn can act as a nucleating seed to trigger the misfolding of the endogenous protein. This process, called “permissive templating”, is thought to occur inside endosomes and lysosomes. 5) Monoclonal antibodies with different strain specificity can bind extracellular aSyn. 6) The immune complex can be internalized by microglia through the FCγRIII and is degraded intracellularly. 7) Unbound monoclonal antibodies can be internalized by neurons through the FCγRI and bind intracellular aSyn. 8) Single-chain variable fragment antibodies or single-domain antibodies (nanobodies) can be delivered as genes with viral vectors. 9) These intrabodies can target intracellular aSyn to prevent aggregation and can be engineered to be more efficient in stimulating degradation. 10) Antibodies can also target cell surface receptors used by aSyn to enter the cells (e.g., LAG3) to prevent entry. (Created with BioRender.com)