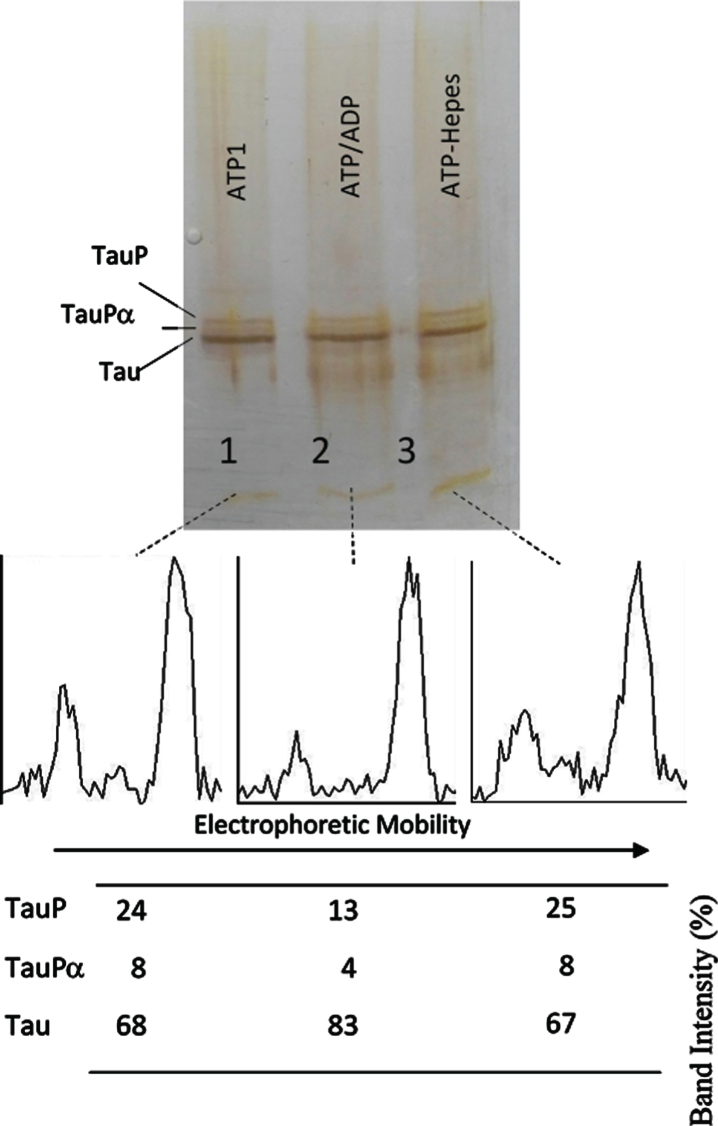
Fig. 6.
Successive incubation of tau protein with ATP and ADP. The mixture of tau protein and C-PKA prepared as described in Materials and Method (Tau-C-PKA) was diluted 1/10 in a buffer composed of 75 μl of Hepes buffer containing 10 mM Hepes and 0.1 M NaCl, at pH 7 and 50 μl of phosphate buffer containing 250 mM phosphate and 0.1 mg/ml Ampicillin at pH 7. This reaction mixture contained 15 mM MgCl2, 0.25 mM EGTA, 50 mM P1, P5-Di(adenosine- 5’)pentaphosphate, 0.25 mM 2-mercaptoethanol, 0.05 mg/ml DNA, and ATP 0.1 mM. After 2 h of incubation at 30°C, in the presence of ATPMg2+, the reaction mixture was divided in three parts: the first one (25 μl) (ATP1) was loaded in the gel (lane 1); the second one (22.5 μl) was incubated at 30°C for 30 min after adding 2.5 ml of 10 mM ADP (ATP/ADP) (lane 2); the third one (22.5 μl) was incubated for the same time, at 30°C, after adding 2.5 μl of Hepes buffer containing 10 mM Hepes, 0.1 M NaCl, 0.1 mg/ml DNA, and 100 μM P1, P5-Di(adenosine- 5’)pentaphosphate at pH 7 (lane 3). The Table at the bottom of the figure shows the results of peak percentages after the scanning of the bands. TauP represent the band displaying the lowest mobility, and TauPα represents the intermediate band usually observed at short times of incubation. The rolling ball radius used for subtraction of background was of 5 pixels.