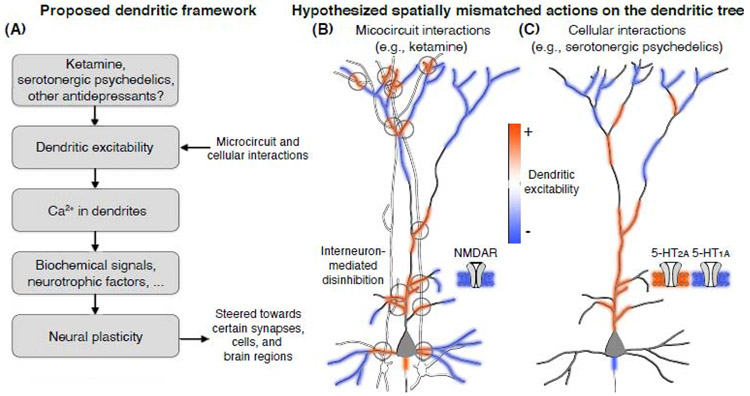
Figure 1. Dendritic excitability as a plausible shared substrate of compounds with fast-acting antidepressant properties.
(A) A flowchart outlining the intuition behind a dendrite-focused framework of antidepressant drug actions: ketamine, serotonergic psychedelics, and potentially other drugs with rapid-acting antidepressant effects could acutely modulate dendritic excitability through idiosyncratic ligand-receptor interactions, inducing local gradients of Ca2+ influx that drive neurotrophic factors (e.g., BDNF) and biochemical cascades (e.g., mTOR) to bias certain synapses for the favorable effects of long-term neural plasticity. Based on this view, schematic illustrations show how (B) ketamine is hypothesized to leverage the microcircuit architecture to drive competing actions on pyramidal cell dendrites: inhibition from NMDAR antagonism directly on pyramidal cells (blue shading) and excitation from interneuron-mediated disinhibition (red shading). By contrast, (C) serotonergic psychedelics may take advantage of compartmentalized distributions of serotonin receptor subtypes to drive competing actions on dendritic excitability: agonism of 5-HT1A receptors, likely along the axonal initial segment or in somato-dendritic distribution, decreases excitability (blue shading), while agonism of 5-HT2A receptors, primarily along the proximal apical dendritic trunk, leads to increased excitability (red shading). The hypothesized spatially mismatched actions illustrated in (B) & (C) are supported by some evidence on interneuron connectivity [62, 63] and receptor localization [41-43, 46, 47], but the scheme remains to be validated.