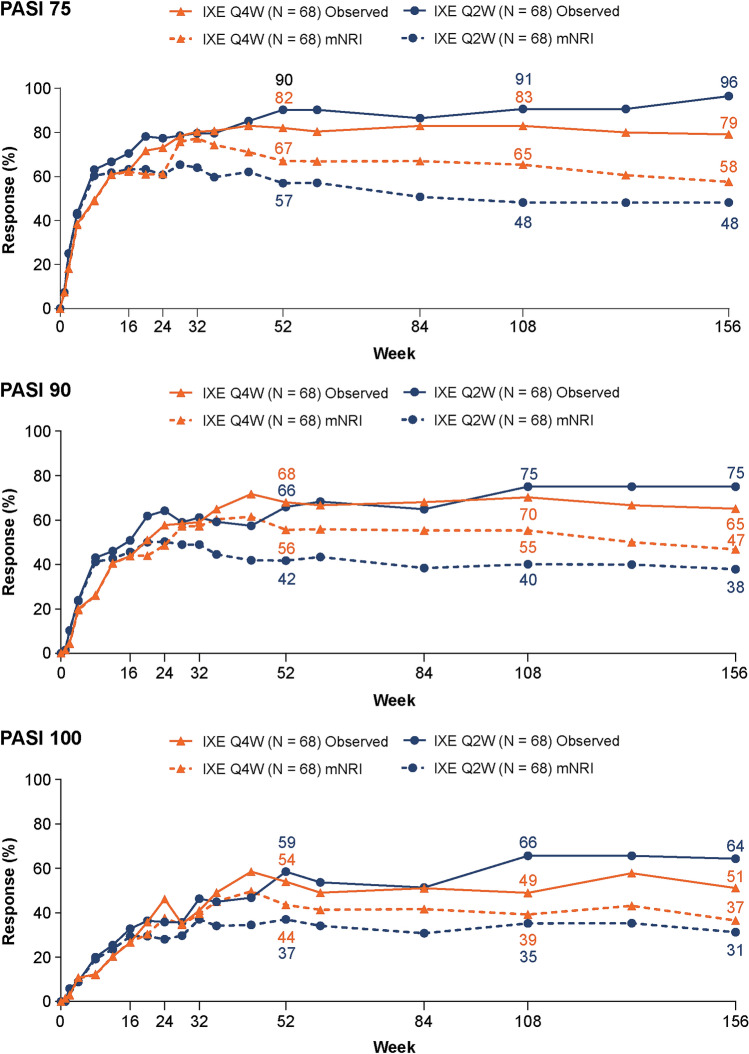
Fig. 3.
Psoriasis Area and Severity Index (PASI) responses (observed and with mNRI). Intent-to-treat population randomized to ixekizumab at week 0 with ≥ 3% body surface area of disease at baseline. Starting at week 32, and at all subsequent visits during the extension period, patients were discontinued from study treatment if they failed to demonstrate ≥ 20% improvement from baseline in tender and swollen joint counts. IXE Q2W ixekizumab 80 mg every 2 weeks, IXE Q4W ixekizumab 80 mg every 4 weeks, PASI 75/90/100 75/90/100% improvement from baseline on the PASI, respectively