Abstract
Adenomyomatosis is a rare benign lesion that has been observed in different sites throughout the gastrointestinal tract, most frequently in the gallbladder. Few cases have been described in the stomach, small bowel, bile ducts, and ampullary region. Adenomyomas of the vaterian system (ampulla and common bile duct) have important clinical consequences, since the majority of these lesions present with biliary tract obstruction and mimic malignant behavior. As a consequence, considering the diagnostic difficulty of these lesions, patients are often treated with extensive surgery (pancreaticoduodenectomy). We report 2 cases of adenomyomatosis: one of the ampulla of Vater and the other of the common bile duct, as well as a review of reported cases in the literature. Both of our patients presented with epigastralgia and had laboratory or endoscopic evidence of biliary obstruction. Both patients underwent endoscopic ultrasound, one of them with fine-needle aspiration; however, it was not possible to exclude the possibility of cancer. The diagnosis of adenomyoma was only confirmed by the surgical specimen after pancreaticoduodenectomy.
Keywords: Adenomyomatous hyperplasia, Adenomyosis, Adenomyomatosis, Adenomyoma, Ampulla of Vater, Common bile duct
Resumo
A adenomiomatose é uma lesão benigna rara que tem sido observada em diferentes locais do trato gastrointestinal, mais frequentemente na vesícula biliar. Poucos casos foram descritos no estômago, intestino delgado, vias biliares e ampola de Vater. Os adenomiomas do sistema de Vater (ampola e via biliar principal) têm importantes consequências clínicas, uma vez que a maioria dessas lesões se apresenta com obstrução biliar, sugerindo comportamento maligno. Como consequência, na maioria dos casos, e considerando a dificuldade diagnóstica destas lesões, os doentes são frequentemente submetidos a cirurgia extensa (pancreaticoduodenectomia). Reportamos dois casos de adenomiomatose da ampola de Vater e via biliar principal, bem como uma revisão dos casos descritos na literatura. Os doentes apresentaram-se com queixas de epigastralgia e evidência laboratorial ou endoscópia de obstrução biliar. Em ambos os casos foi realizada ultrassonografia endoscópica e em um deles punção aspirativa poragulha fina, não tendo sido possível excluir a possibilidade de malignidade. O diagnóstico de adenomioma foi apenas confirmado na peça cirúrgica após pancreaticoduodenectomia.
Palavras Chave: Hiperplasia adenomiomatosa, Adenomiose, Adenomiomatose, Adenomioma, Ampola de Vater, Via biliar principal
Introduction
Adenomyomatosis (adenomyomatous hyperplasia, adenomyosis, or adenomyoma) is a rare benign lesion that has been observed in different sites throughout the gastrointestinal tract, most frequently in the gallbladder [1]. Adenomyomatosis of the gallbladder is most often an incidental finding during cholecystectomy performed for another reason with a prevalence of 1–9%, and large autopsy series report a prevalence of 7% [2, 3]. Few cases have been described in the stomach, small bowel, bile ducts, and ampullary region. Adenomyomas of the vaterian system (ampulla of Vater [AV] and common bile duct [CBD]), unlike its counterparts in the rest of the digestive tract, have important clinical consequences, since the majority of these lesions present with biliary tract obstruction and mimic malignant behavior [1]. As consequence, despite being a benign lesion in most cases, patients are often treated with extensive surgery (pancreaticoduodenectomy). We report 2 cases of adenomyomatosis: one of the AV and the other of the CBD, as well as a review of cases reported in the literature. Both of our patients presented with epigastralgia and had laboratory or endoscopic evidence of biliary obstruction. The diagnosis of adenomyoma was only confirmed by the surgical specimen after cephalic pancreaticoduodenectomy.
Case Reports
Case 1
A 70-year-old woman with previous laparoscopic cholecystectomy for gallstones and a history of hypertension and dyslipidemia was referred to a gastroenterologist for epigastralgia and an abnormal abdominal CT scan, which revealed CBD dilatation (22 mm) with progressive reduction in size, without any AV or pancreas distortion. She had no family history of cancer and no jaundice. Laboratory workup showed elevated transaminases: 72 IU/L aspartate transaminase (AST) and 86 IU/L alanine transaminase (ALT). Alkaline phosphatase (ALK), γ-glutamyltransferase (GGT), total and conjugated bilirubin, and amylase were within normal ranges. Carcinogen antigen 19.9 (CA 19.9) was normal. She had a normal complete blood count and no elevation in acute-phase reactants (Table 1). A magnetic resonance cholangiopancreatography (MRCP) confirmed CBD dilatation with a localized stenosis 1 cm above the ampulla. A subsequently performed endoscopic ultrasound (EUS) showed a dilated CBD (16 mm) and a poorly defined hypoechogenic mass (1.5 × 1.9 cm) in the distal part. There was neither main pancreatic duct (MPD) or parenchyma involvement nor evidence of lymph node, ascites, or left hepatic lobe alterations (Fig. 1). A duodenoscopy showed a bulging AV with normal mucosa (Fig. 2). EUS-guided fine-needle aspiration (FNA) or brush cytology/biopsies obtained by endoscopic retrograde cholangiopancreatography (ERCP) was not performed because a negative or inconclusive histology would not change our therapeutic approach, since malignancy suspicion was high. The case was discussed at a digestive oncology multidisciplinary meeting and in consideration of the diagnostic hypothesis of cholangiocarcinoma of the distal bile duct and after discussion with the patient, she was submitted to a cephalic pancreaticoduodenectomy, which was performed 1 month later. Surgery was uneventful, and the patient was discharged on the 15th postoperative day. Macroscopic examination of the surgical specimen showed a bulging AV, CBP dilatation, and a subepithelial lesion without duodenal wall or pancreas invasion (Fig. 3). Histologically, the lesion consisted of hyperplastic glandular lobules surrounded by muscle fibers and fibroblasts, suggestive of adenomyomatosis of the CBP and AV (Fig. 4). At the 3-year follow-up, she was asymptomatic and without laboratory abnormalities.
Table 1.
Full blood workup (case 1)
| Parameter | Value |
|---|---|
| Hemoglobin, g/dL | 13.0 |
| White blood cells, ×109/L | 5,000 |
| Platelets, ×109/L | 331,000 |
| Urea, mg/dL | 18 |
| Creatinine, mg/dL | 0.67 |
| AST, IU/L | 72 |
| ALT, IU/L | 86 |
| ALK, IU/L | 47 |
| GGT, IU/L | 26 |
| Total bilirubin, mg/dL | 0.26 |
| Conjugated bilirubin, mg/dL | 0.17 |
| Amylase, IU/L | 70 |
| Serum sodium, mg/dL | 140 |
| Serum potassium, mg/dL | 4 |
| C-reactive protein, mg/dL | 0.1 |
| CA 19.9 | 6.9 |
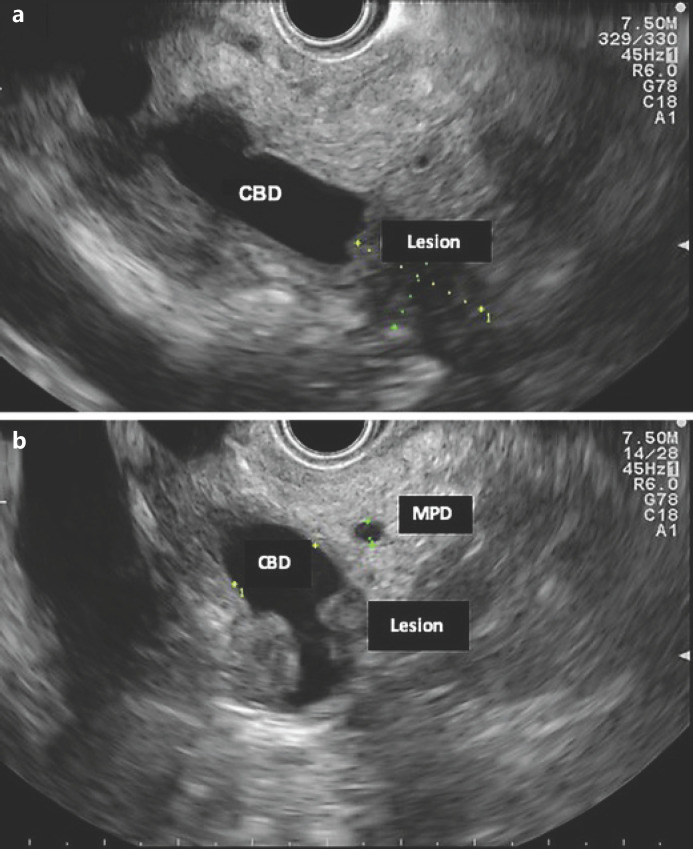
Fig. 1.
EUS (linear endoscope) reveals dilated CBD and a poorly defined hypoechogenic mass in its distal portion (a). b Mass in the distal common bile duct.
Fig. 2.

Duodenoscopy showing bulging of the ampulla of Vater with a normal mucosa.
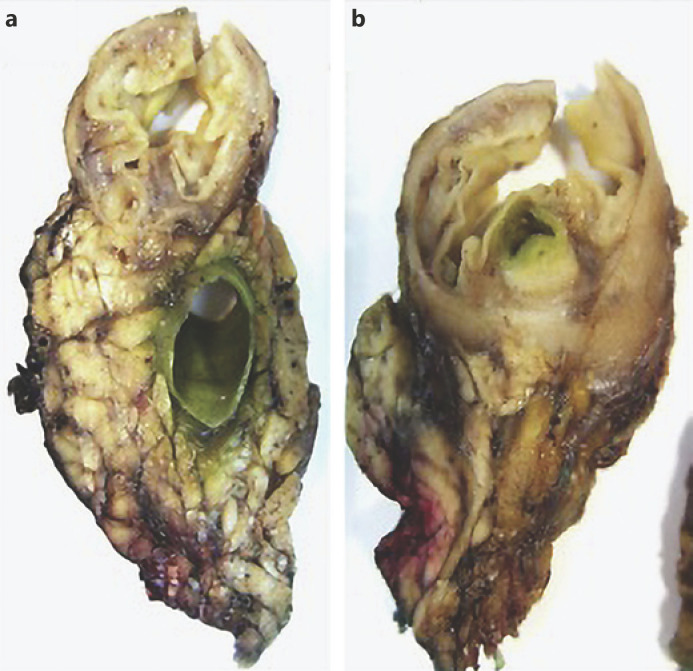
Fig. 3.
Macroscopic examination of a surgical specimen shows bulging of the ampulla and CBD dilatation (a) and a subepithelial lesion without duodenal wall or pancreas invasion (b).
Fig. 4.
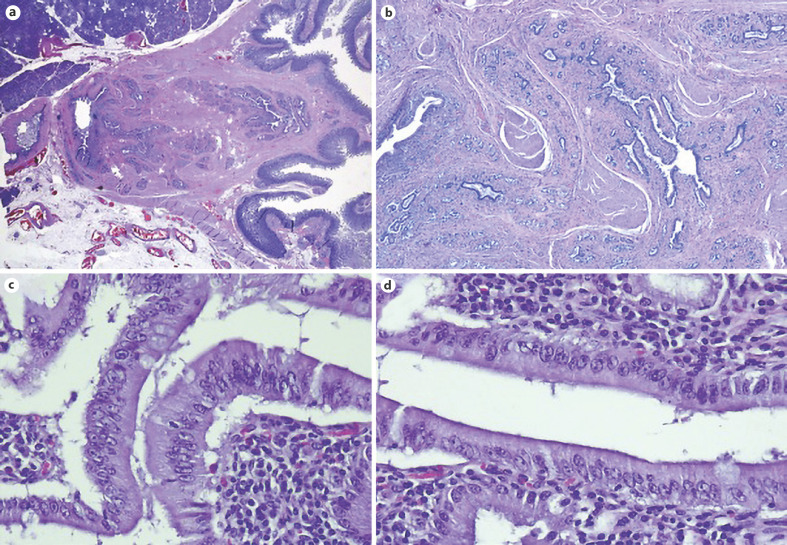
Microscopic examination of surgical specimens. H&E. a. Low magnification with subepithelial lesion. b ×4. c Hyperplastic glandular lobules surrounded by muscle fibers and fibroblasts. ×10.
Case 2
A 58-year-old man with a history of peptic ulcer disease and gastroesophageal reflux was referred to a gastroenterologist after an upper GI endoscopy, performed for epigastralgia. A protruding ampulla with a normal mucosa was described (Fig. 5). He had no family history of cancer and had no jaundice. Laboratory workup showed elevated transaminases with AST of 52 IU/L and ALT of 64 IU/L. ALK, GGT, total and conjugated bilirubin, and amylase were within normal ranges. A complete blood count was normal, and acute-phase reactants were not elevated. CA 19.9 was normal (Table 2). EUS showed a 12-mm, poorly defined, hypoechogenic mass in the AV area, with involvement of the distal CBD and the muscular layer of the duodenal wall. There was no evidence of CBD dilatation or pancreatic involvement (Fig. 6). FNA was performed. Cytological examination showed groups of epithelial cells: some with benign characteristics and others with nuclear overlap and increased nuclei, in favor of epithelial dysplasia, without obvious carcinoma characteristics (Fig. 7). The case was discussed at a digestive oncology multidisciplinary meeting; considering the diagnostic hypothesis of ampulloma and after discussing the case with the patient, she was submitted to cephalic pancreaticoduodenectomy, which was performed 1 month later. The surgery was uneventful, and the patient was discharged on the 8th postoperative day. Macroscopic examination of the surgical specimen showed a white and firm tumor of 1.6 cm in largest diameter (Fig. 8). Histologically (Fig. 9), there was a slight CBD and MPD dilatation and some inflammatory infiltrate. The ampulla consisted of aggregates of ductal proliferation surrounded by fibrosis, which had continuity with the muscular layer of the duodenal wall. There were some areas with enlarged and stratified nuclei in favor of reactive atypia. These findings were consistent with the diagnosis of AV adenomyomatosis. At the 2-year follow-up, she was asymptomatic and without analytical alterations.
Fig. 5.
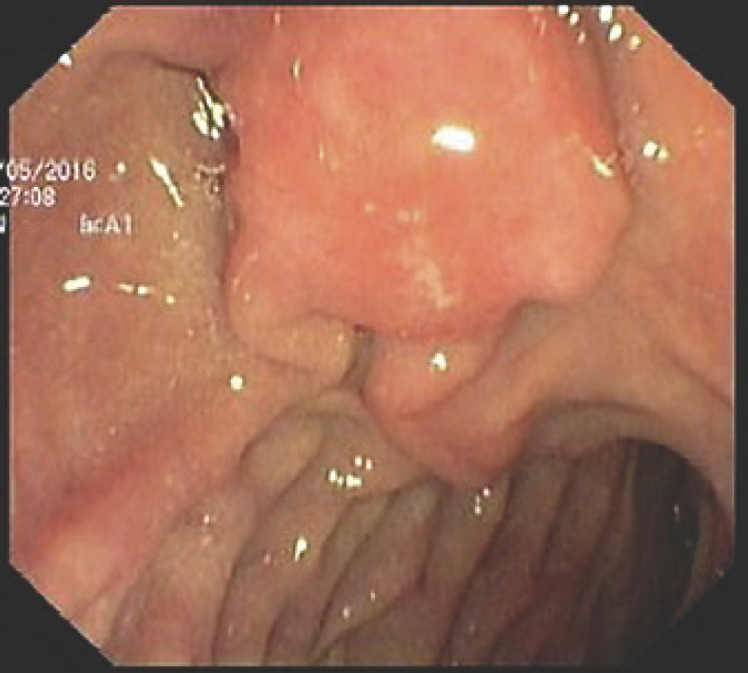
Duodenoscopy with protruding ampulla with a normal mucosa.
Table 2.
Full blood workup (case 2)
| Parameter | Value |
|---|---|
| Hemoglobin, g/dL | 15.2 |
| White blood cells, ×109/L | 6,000 |
| Platelets, ×109/L | 219,000 |
| Urea, mg/dL | 18 |
| Creatinine, mg/dL | 0.83 |
| AST, IU/L | 52 |
| ALT, IU/L | 64 |
| ALK, IU/L | 40 |
| GGT, IU/L | 21 |
| Total bilirubin, mg/dL | 0.44 |
| Conjugated bilirubin, mg/dL | 0.12 |
| Amylase, IU/L | 90 |
| Serum sodium, mg/dL | 136 |
| Serum potassium, mg/dL | 3.9 |
| C-reactive protein, mg/dL | 0.2 |
| CA 19.9 | 1.3 |
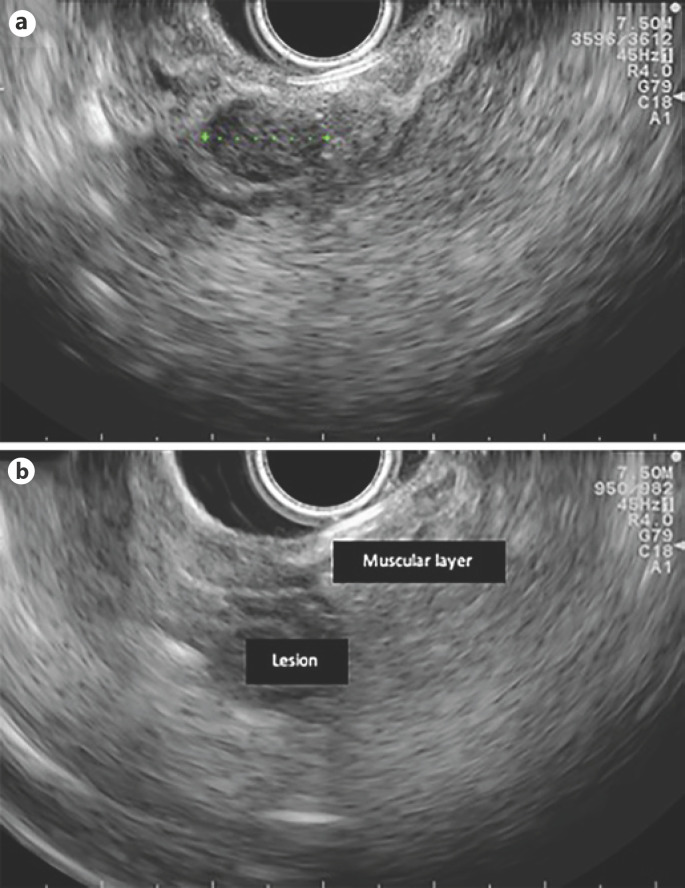
Fig. 6.
EUS features (linear endoscope): 12-mm hypoechogenic mass in the ampulla area (a) and a lesion with duodenal-wall muscular-layer involvement (b).
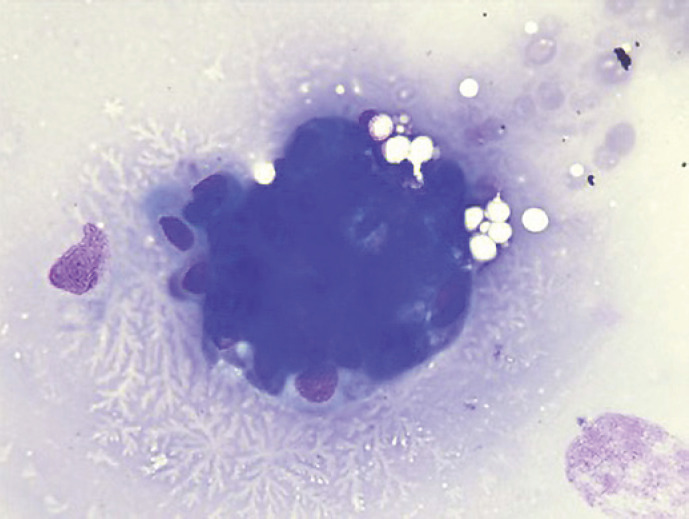
Fig. 7.
Cytology examination showed groups of epithelial cells: some with benign characteristics and others with nuclear overlap and increased nuclei in favor of epithelial dysplasia without obvious carcinoma characteristics.
Fig. 8.
Macroscopic examination of a surgical specimen: bulging ampulla (a) and white and firm tumor 1.6 cm in the largest diameter (b).
Fig. 9.
Microscopic examination of the specimen. H&E. a Low magnification showing that the lesion consisted of aggregates of ductal proliferation surrounded by fibrosis and had continuity with the muscular layer of the duodenal wall. b Low magnification demonstrates slight dilatation of CBD and MPD, and chronic periductal inflammatory infiltrate. c, d Areas with enlarged and stratified nuclei in favor of reactive changes. ×40.
Discussion
According to the WHO classification, adenomyoma is a benign lesion with no premalignant risk, defined as duct-like structures accompanied by hyperplasia of smooth muscle bundles [4]. The real incidence of these lesions is difficult to settle as different names are used to designate the same histological lesion [1]. Published series of unselected postmortem examinations report an incidence of 50–70% of small adenomyomas of the vaterian system (<5 mm), a high percentage of cases having no relevant associated clinical history. Symptomatic lesions reported in the medical literature are much rarer and reported mostly as single case reports [5, 6]. To our knowledge, this is the first literature review regarding AV and CBD adenomyomatosis. Our 2 cases were a 70-year-old woman and 58-year-old man with laboratory or endoscopic evidence of biliary obstruction, in whom preoperative diagnosis was ambiguous, and the diagnosis of adenomyoma was only confirmed by the surgical specimen after pancreaticoduodenectomy.
A PubMed search conducted using the key words “adenomyomatous hyperplasia,” “adenomyoma,” “adenomyosis,” “adenomyomatosis,” and “ampulla of Vater” or “common bile duct,” revealed 61 case reports (from 1987 to July 2018) eligible for analysis (Table 3). Regarding published cases, almost half of the patients were male (n = 29, 48%), and their mean age was 62 years (range 18–81 years). Forty-nine patients had AV adenomyoma (80%), and 12 had CBD adenomyoma (20%). Patients presented with jaundice (n = 22/61), abdominal pain (n = 25/61), nausea and vomiting (n = 3/61), acute pancreatitis (n = 2/61) − both with AV lesions, loss of appetite (n = 3/61), and fatigue (n = 1). Fifteen patients (25%) were asymptomatic, and the finding was incidental. For 1 patient, clinical presentation was not mentioned. Nineteen patients had cholestasis/conjugated hyperbilirubinemia, 10 had transaminase, ALK, or GGT elevation with normal bilirubin, 2 patients had elevated amylase and lipase, and 3 patients had normal liver tests. In 26 cases, laboratory workup was not reported. Imaging (abdominal CT, MRI, and MRCP) and endoscopic (ERCP and upper GI EUS) features more frequently found were CBD or MPD dilation, tumor-like mass in the papilla region or distal CBD, CBD stenosis, intrahepatic biliary tract dilation, and bulging papilla (in patients with ampullary lesions). There was no preoperative or intraoperative histological diagnosis in 26 patients. In the other patients, several different diagnoses were made: 2 adenomas, 5 adenocarcinomas, 2 cases of inflammatory changes, 3 cases of dysplasia, 3 cases of atypical cells, 3 cases of muscular and glandular proliferation, 1 case of suspected adenomyoma, 8 adenomyomas, 1 adenomyoma with dysplasia, and 1 patient without malignant cells. Besides these pre-/intraoperative diagnoses, in 6 patients, intraoperative frozen sections revealed adenomyomatosis of ampullary, glandular, and muscular proliferation, muscle-cell hyperplasia, uncertain for malignancy, atypical cells, and negative for malignancy, respectively. Consequently, only in 9 patients (15%), adenomyoma was diagnosed pre-/intraoperatively. These patients were submitted to endoscopic papillectomy (n = 4), surgical papillectomy (n = 1), and close observation (n = 4). Forty-one patients (67%) underwent duodenopancreatectomy, 7 patients were submitted to endoscopic ampullectomy, 2 patients underwent surgical ampullectomy, 2 had local surgical/extensive excision, 2 had CBD surgical resection, 1 had endoscopic mass excision using biopsy forceps, and 4 patients received close observation with repeated endoscopic observations (lesions did not change with time, but the duration of follow-up time is mentioned in the case report).
Table 3.
Literature review of adenomyomatosis of the ampulla of Vater (AV) and common bile duct (CBD)
| Reference | Age, sex | Location | Clinical presentation | Laboratory changes | Imaging/endoscopic features | Pre-/intraoperative histologic diagnosis | Treatment/ management |
|---|---|---|---|---|---|---|---|
| Uljch et al. [20], 1987 | Elderly, F | AV | NA | Biliary obstruction | Recent onset of CBD dilatation | Not made | Extensive surgical resection |
| Ikei et al. [21], 1989 | 71, F | CBD | Jaundice, fever and abdominal pain | Conjugated hyperbilirubinemia (6.8 mg/dL), |
CT: dilation of intra- and extrahepatic duct; PTC: dilation of CBD and stenosis of its | Not made | PD |
| ↑ ALK (123 IU/L), ↑ AST (54 IU/L), ↑ ALT (95 IU/L) | distal part | ||||||
| Ikei et al. [21], 1989 | 52, F | CBD | Abdominal pain | Normal | ERCP: dilated CBD (17 mm) with mural irregularity (about 26 mm long) and a spherical immobile shadow defect at the end of the bile duct | Not made | PD |
| Legakis et al. [22], 1990 | 55, F | CBD | Jaundice and abdominal pain |
Conjugated hyperbilirubinemia (5.7 mg/dL), ↑ ALP (1,700 IU/L) | Not made | Not made | Choledochal excision and hepaticojejunal anastomosis |
| Läuffer et al. [23], 1998 | 69, F | CBD | Abdominal pain | Normal | ERCP: hemispherical contrast defect in the terminal CBD 1.5 cm proximal to the papilla of Vater | Biopsies: suggestive of adenoma, but not conclusive; brush cytology: no malignant cells; frozen sections indicated benign hyperplasia of muscle cells without evidence of malignancy | Surgical local tumor excision in the distal CBD through the papilla of Vater |
| Narita et al. [19], 1999 | 73, F | AV | Jaundice | NA | US: dilated CBD (15 mm); CT scan showed a tumor-like mass in the papilla of Vater | Not made | CPD |
| Kalil et al. [24], 2000 |
18, M | AV | Jaundice and abdominal pain |
Conjugated hyperbilirubinemia (26 mg/dL), ↑ AST (87 IU/L), ↑ ALT (53 IU/L), ↑ GGT (247 IU/L) |
CT: dilation of CBD (17 mm), IOC: CBD dilation and a periampular mass (20 mm) | Not made | CPD |
| Tsukamoto et al. [25], 1999 | 31, F | CBD | Epigastralgia | Cholestasis | Stenosis of the CBD | Not made | Resection of the CBD with choledochojejunostomy |
| Ojima et al. [26], 2000 | 64, F | CBD | Jaundice, abdominal pain, fever and appetite loss | NA | ERCP: possible stenosis in the distal CBD | Not made | DP |
| Kayahara et al. [27], 2001 | 42, F | AV | Abdominal pain | ↑ AST (63 IU/L), ↑ ALT (113 IU/L), ↑ GGT (173 IU/L) | ERCP: abnormal shadow in the periampullary region; PTC: choledochal obstruction; IOC: lack of contrast passage to duodenum and CBD dilation | Papilla biopsy: mucosal hyperplasia; intraoperative frozen section: adenomyomatosis of the ampulla | Surgical papillotomy and sphincteroplasty |
| Bedirli et al. [28], 2002 |
63, M | AV | Epigastric pain | Conjugated hyperbilirubinemia (4.2 mg/dL), ↑ ALP (397 IU/L) | CT: dilated CBD and an AV mass (2.5 × 2.0 cm); ERCP: CBD and MPD dilation |
Intraoperative frozen section ruled out malignancy | DP |
| Bedirli et al. [28], 2002 |
51, M | AV | Jaundice | Conjugated hyperbilirubinemia (11.7 mg/dL), ↑ ALP (958 IU/L)), ↑ AST (60 IU/L), ↑ ALT (92 IU/L) | CT: intrahepatic biliary dilation and dilated CBD (17 mm), possible mass in the head of the pancreas | Not made | DP |
| Handra-Luca et al. [1], 2003 | 64, M | AV | Abdominal pain | NA | Heterogenous intra-ampullary lesion (11 mm) | Brush cytology: atypical cells | DP |
| Handra-Luca et al. [1], 2003 | 61, M | AV | Jaundice and epigastric pain | NA | No lesion | Adenoma | DP |
| Handra-Luca et al. [1], 2003 | 73, M | AV | Asymptomatic | NA | Intra-ampullary lesion (21 mm) | Inflammatory changes | DP |
| Handra-Luca et al. [1], 2003 | 67, F | AV | Jaundice | NA | Intra-ampullary lesion (15 mm) | Adenocarcinoma | DP |
| Handra-Luca et al. [1], 2003 | 54, F | AV | Asymptomatic | NA | Intra-ampullary lesion (20 mm) | Adenoma | DP |
| Handra-Luca et al. [1], 2003 | 55, M | AV | Asymptomatic | Cholestasis | Hypoechogenic intra-ampullary lesion (15 mm) | Severe dysplasia | DP |
| Handra-Luca et al. [1], 2003 | 71, F | AV | Right upper quadrant pain | NA | Hyperechogenic intra-ampullary lesion (10 mm) | Muscle fibers, glandular structures | DP |
| Handra-Luca et al. [1], 2003 | 78, F | AV | Jaundice | Cholestasis | Tumor located in the pancreas head | Not made | DP |
| Handra-Luca et al. [1], 2003 | 49, F | AV | Right upper quadrant pain | NA | NA | Hyperplastic glandular structures, inflammatory changes | DP |
| Handra-Luca et al. [1], 2003 | 38, F | AV | Abdominal pain | NA | NA | Low-grade dysplasia | DP |
| Handra-Luca et al. [1], 2003 | 67, M | AV | Jaundice | NA | Thickening of the terminal CBD wall | Not made | DP |
| Handra-Luca et al. [1], 2003 | 74, M | AV | Jaundice | NA | Heterogenous intra-ampullary lesion (20 mm) | Not made | DP |
| Handra-Luca et al. [1], 2003 | 72, F | AV | Asymptomatic | NA | Intra-ampullary lesion (10 mm) | Adenocarcinoma | DP |
| Aoun et al. [16], 2005 | 68, M | AV | Jaundice and epigastric pain | NA | CT: hypoattenuating lesion in the ampullary region slightly indenting the duodenal lumen (17 × 20 mm) | Not made | CPD |
| Aoun et al. [16], 2005 | 71, M | AV | Right upper-quadrant pain | NA | CT: hypoattenuating lesion in the ampullary region (10 × 10 mm) and a dilated pancreatic duct; ERC: distal CBD stenosis | Not made | CPD |
| Aoun et al. [16], 2005 | 66, F | AV | Epigastric pain | NA | CT: 14 × 10 mm lesion protruding into the duodenal lumen | Not made | CPD |
| Martinez Vieira et al. [29], 2005 | 22, M | AV | Jaundice and weight loss | Conjugated hyperbilirubinemia (4.5 mg/dL), ↑ AST (122 IU/L) | CT: dilated CBP (10 mm) with a stop sign 2 cm above the ampulla; EUS: bulging ampulla, stenosis of distal CBP |
Brush cytology: negative for malignancy; intraoperative biopsies: cell of uncertain malignancy |
CPD |
| Massom et al. [30], 2006 | 73, F | AV | Nausea, vomiting and diarrhea | NA | CT: oval cystic lesion (1.8 × 1.2 cm) in the uncinate process of the pancreas, surrounded by normal-appearing pancreatic tissue | EUS with targeted biopsy: 2 cell populations, one composed of benign-appearing duct-epithelial cells and the other consisting of an occasional focus of atypical cells with loss of polarity and necrosis | CPD |
| Kwon et al. [31], 2007 | 74, F | AV | Acute recurrent pancreatitis |
↑ AST (47 IU/L), ↑ ALT (49 IU/L), ↑ amylase (4,290 IU/L), lipase (1,526 IU/L) | MRCP: dilated CBD, with distal stenosis; ERCP: bulging papilla, after sphincterotomy, a nodular mass with a granular and villous mucosa originating from the peripancreatic orifice |
Muscle proliferation without atypia | Piecemeal endoscopic resection of the mass with electrocautery snare and coagulation of the remnant villous mucosa with argon plasma |
| Shu et al. [32], 2008 |
51, M | CBD | Jaundice, nausea, and weight loss | ↑ ALT (250 IU/L), ↑ AST (102 IU/L), ↑ ALK (641 IU/L), ↑ GGT (854 IU/L), total bilirubin (1.9 mg/dL) | MRCP: dilatation of the CBD and pancreas duct, irregular stricture of the bile duct and a 1.6 × 0.27 cm mass at pancreatic segment of CBD | Not made | DP |
| Iwaki et al. [33], 2008 | 62, F | CBD | Asymptomatic | ↑ ALK | CT: thickening of the lower CBD wall; ERCP: a 15-mm-long stenosis of the lower CBD | Bile/brush cytology: no malignant cells | DP |
| Genevay et al. [34], 2009 | 73, M | AV | Jaundice | ↑ ALK (997 IU/L), ↑ bilirubin (17 mg/dL) | CT: dilated intrahepatic bile ducts; ERCP: 40-mm-long CBD stricture | Not made | DP |
| Lehwald et al. [35], 2010 | 42, M | AV | Nausea and vomiting | CA 19.9 normal | CT: a mass in the pancreas head and duodenum with duodenal and distal bile duct stenosis | Intraoperative frozen section: atypical cells | DP |
| Higashi et al. [36], 2010 | 67, F | AV | Jaundice | NA | NA | Suspected adenomyoma | DP |
| Higashi et al. [36], 2010 | 78, F | AV | Asymptomatic | NA | NA | Not made | DP |
| Higashi et al. [36], 2010 | 49, M | AV | Asymptomatic | NA | NA | Vater carcinoma | DP |
| Higashi et al. [36], 2010 | 58, F | AV | Jaundice | NA | NA | Vater carcinoma | DP |
| Higashi et al. [36], 2010 | 68, M | AV | Jaundice | NA | NA | Vater carcinoma | DP |
| Kumari et al. [37], 2011 | 58, M | AV | Abdominal pain | NA | CBD dilatation | Not made | DP |
| Kumari et al. [37], 2011 | 65, M | AV | Jaundice | NA | CBD dilatation | Not made | DP |
| Kumari et al. [37], 2011 | 81, M | AV | Appetite loss | NA | CBD dilatation and nodule at ampulla (1.5 × 1 cm) | Not made | DP |
| Choi et al. [38], 2013 | 70, M | AV | Abdominal pain | ↑ ALT (78 IU/L) ↑ ALK (268 IU/L) | CT: ampullary lesion and CBD dilation (14 mm); endoscopy: ampullary mass with granularity (15 mm) | Endoscopic biopsy: adenomyoma | Endoscopic papillectomy |
| Choi et al. [38], 2013 | 71, M | AV | Abdominal pain | ↑ ALT (55 IU/L) ↑ AST (92 IU/L) ↑ ALK (432 IU/L) ↑ total bilirubin (1.83 mg/dL) | Endoscopy: ampullary mass (12 mm) | Endoscopic biopsy: adenomyoma | Endoscopic papillectomy |
| Choi et al. [38], 2013 | 72, M | AV | Asymptomatic | ↑ ALK 180 | CT: well-defined nodule at ampulla (15 × 12 mm) and CBD dilatation (13 mm) | Endoscopic biopsy: dysplasia, adenomyoma | Endoscopic papillectomy |
| Choi et al. [38], 2013 | 53, M | AV | Abdominal pain | ↑ ALK (300 IU/L) ↑ total bilirubin (1.46 mg/dL) | Endoscopy: lobulated lesion of the ampulla (10 mm) | Endoscopic biopsy: chronic inflammation, adenomyoma | Endoscopic papillectomy |
| Choi et al. [38], 2013 | 75, M | AV | Asymptomatic | ↑ ALK (230 IU/L) | CT: focal enhancing lesion at ampulla with CBD dilatation (11 mm); endoscopy: bulging and lobulated papilla | Endoscopic biopsy: adenomyoma | Surgical ampullectomy |
| Choi et al. [38], 2013 | 75, F | AV | Asymptomatic | ↑ ALK (251 IU/L) | 11-mm mass of the ampulla and CBD dilatation (10 mm); endoscopy: bulging and lobulated papilla |
Endoscopic biopsy: adenomyoma | Close observation |
| Choi et al. [38], 2013 | 64, F | AV | Asymptomatic | ↑ ALK (178 IU/L) | Endoscopy: CBD dilatation (10 mm) and bulging papilla | Endoscopic biopsy: adenomyoma | Close observation |
| Choi et al. [38], 2013 | 57, F | AV | Asymptomatic | ↑ ALK (174 IU/L) ↑ total bilirubin (1.02 mg/dL) | Endoscopy: enlarged and lobulated papilla | Endoscopic biopsy: atypical epithelial proliferation − adenomyoma | Close observation |
| Choi et al. [38], 2013 | 65, F | AV | Asymptomatic | ↑ ALK (273 IU/L) | Endoscopy: bulging and lobulated papilla | Endoscopic biopsy: adenomyoma | Close observation |
| Rafiullah et al. [39], 2014 | 61, M | AV | Acute pancreatitis | ↑ AST (190 IU/L), ↑ ALT (169 IU/L), ↑ ALK (147 IU/L), ↑ amylase (1,855 IU/L), ↑ lipase (285 IU/L) | CT: acute pancreatitis with a peripancreatic adenopathy, dilatation of CBD (10 mm) with abrupt discontinuation at the pancreas head and mildly dilated pancreatic duct (4 mm) at the pancreas head; EUS: multilobulated hypoechoic ampullary density (2.4 × 2.1 cm) | EUS-FNA: reactive cells; endoscopic biopsies: inflammatory polyp versus inflammatory changes overlying an unsampled neoplastic lesion | Endoscopic ampulectomy |
| Choi et al. [40], 2016 | 42, M | CBD | Jaundice, epigastric pain and vomiting | Conjugated hyperbilirubinemia (7.8 mg/dL), ↑ ALK (689 IU/L), ↑ GGT (1,199 IU/L), ↑ AST (224 IU/L), ↑ALT (266 IU/L) |
CT: abrupt narrowing of the distal CBD and proximal bile duct dilatation | Endobiliary biopsies: chronic inflammation with fibrosis, periductal glandular proliferation, dysplastic change; slightly positive for p53 |
DP |
| D'Assuncao et al. [41], 2016 | 50, F | CBD | Abdominal pain | Liver tests were normal | MRI: dilated CBD; EUS: hypoechoic lesion near the papilla (5.2 mm) |
Not made | Endoscopic excision using biopsy forceps |
| Keegan et al. [42], 2017 | 59, M | AV | Fatigue | ↑ AST (160 IU/L), ↑ ALT (61 IU/L), ↑ GGT (63 IU/L) | CT: hypodensity (10 mm) at the AV with prominent extrahepatic biliary duct; duodenoscopy: a bulky ampulla; EUS: dilated extrahepatic biliary duct (11 mm) with hypoechoic ampullary lesion (13.5 mm) | Not made | Endoscopic ampullectomy |
| Gialamas et al. [43], 2018 | 73, F | AV | Jaundice and fatigue | ↑ AST (1,008 IU/L), ↑ ALT (1,105 IU/L), ↑ ALK (153 IU/L), ↑ GGT (114 IU/L), conjugated hyperbilirubinemia (21.6 mg/dL) | MRCP: stenosis of the distal CBD at AV level, with dilatation (10 mm) above this region; EUS: retro-ampullary mass |
Ampullary mass biopsies: atypical cells and chronic inflammation of the ampulla, without dysplasia | CPD |
| Gouveia et al., 2019 | 58, M | AV | Epigastralgia | ↑ AST (52 IU/L), ↑ ALT (64 IU/L) | Endoscopy: bulging papilla; EUS: mass (12 mm) in the ampulla area, with distal CBD and apparently duodenal wall muscular involvement | EUS-FNA: epithelial cell groups, some with benign characteristics, others with nuclear overlap and increased nuclei in favor of epithelial dysplasia, without obvious carcinoma characteristics | CPD |
| Gouveia et al., 2019 | 70, F | CBD | Epigastralgia | ↑ AST (72 IU/L), ↑ ALT (86 IU/L) | CT: CBD dilatation (22 mm) with progressive reduction in size; MRCP: CBD dilatation with a localized stenosis 1 cm above the ampulla; EUS: dilated CBD (16 mm) and a poorly defined hypoechogenic mass (1.5 × 1.9 cm) in the distal CBD | Not made | CPD |
CPD, cephalic pancreaticoduodenectomy; DP, duodenopancreatectomy; IOC, intraoperative cholangiography; PTC, percutaneous transhepatic cholangiography.
The diagnosis of adenomyoma of the vaterian system (AV and CBD) is challenging. Patients often present with signs of biliary obstruction and cholestasis, and preoperative imaging (CT, MRI, and MRCP) frequently shows common bile duct obstruction or a tumor-like mass. Endoscopic biopsies, EUS-FNA and brush cytology show most of the time atypical cells, dysplasia, or even malignancy. In retrospect, these findings are thought to be secondary to AV and CBD endoscopic manipulation (biopsy, brush cytology, and sphincterotomy), and may contribute to the diagnostic difficulties. The overall accuracy for preoperative histopathological diagnosis with endoscopic forceps biopsies in patients with AV tumors was reported as 62% by Menzel et al. [7]. Hammarström et al. [8], in a study including 3,131 patients submitted to ERCP, showed that a correct endoscopic diagnosis was only made in 2 of the 4 patients with adenomyoma. ERCP also allows for brush cytology and intraductal biopsy performance. The sensitivity of brush cytology and intraductal biopsy in diagnosing malignant biliary strictures are reported as 45 and 48.1% respectively, and both techniques are almost 100% specific. A combination of both modalities modestly increased the sensitivity to 59.4% [9]. To overcome this limitations, Kim et al. [10] and Uchida et al. [11] showed that repeated testing (multiple cytology tests) via endoscopic nasobiliary drainage increased the cumulative diagnostic rate, with a sensitivity of 95% with 6 repeated exams [10, 11]. Logrono et al. [12], who analyzed 183 pancreatobiliary brush specimens from 2 university hospitals, showed that the possibility of malignancy with no evidence of malignancy from repetitive endoscopic biopsy was lower than 10%. EUS-FNA can be performed for distal extrahepatic bile duct strictures, with a sensitivity and negative likelihood ratio for diagnosis of malignancy of 66% and 0.34, respectively [13]. Furthermore, EUS-FNA can be performed in ampullary and distal CBD masses with an overall accuracy of 100%, with a sensitivity, specificity, and positive and negative predictive values of 100% [14]. Intraoperative frozen sections from the mass can usually differentiate whether the lesion is benign or malignant (adenomyoma and adenocarcinoma). However, most pathologists have limited experience with frozen-section adenomyomas [15]. Macroscopically, adenomyoma of the ampullary region usually appears as a rounded, well-defined, intraluminal lesion arising from the CBD wall, although some case reports have described a diffuse form infiltrating the CBD wall which resembles a stenotic lesion [16]. The histological aspect of adenomyoma is characterized by multiple lobules of glands, mainly located in the muscle layers of the vaterian system. The lobular formations consist of small glands arranged around a larger gland and surrounded by myofibroblastic and fibroblastic proliferation. This mesenchymal component is rather composed of fibroblasts and myofibroblasts (with smooth muscle actin expression but without desmin expression), but it may contain sparse smooth muscle cells [1]. The histogenesis of adenomyoma and adenomyomatous hyperplasia is still a subject of controversy. The most widely accepted hypothesis is that these lesions may represent a form of incomplete heterotopic pancreas (type III), as described by von Heinrich in 1909 [1]. The presence of hyperplastic smooth muscle tissue can be explained by secondary muscle proliferation caused by some stimulus emanating from misplaced epithelium, by muscle misarrangement, or by aberrant growth invading and distorting normal muscle [1]. Martin et al. [17] compared adenomyoma of the vaterian system to its gallbladder counterpart and claimed that the former is a lesion developed in diverticula, accompanied by reactive muscle hyperplasia and secondary gland formation, which leads to poorly defined lobules. Fernandez-Cruz and Pera [18] considered adenomyoma as part of an involutive process of fibroadenomatous type due to increasing age. Other authors, such as Narita and Yokoyama [19], stress the possibly inflammatory nature of this lesion.
Conclusion
Adenomyomatosis of CBD and AV are rare benign lesions, which pose a diagnostic challenge, as they often present with biliary obstruction and mimic malignant neoplasms; imaging and endoscopy rarely offer a definitive diagnosis. As a consequence, in most cases, patients are treated with extensive surgery despite its benign nature. The development and application of new endoscopic, radiological, and pathological modalities are necessary in order to improve the diagnosis and management of these lesions.
Statement of Ethics
The patients have given written informed consent to publish the details of their case (including the publication of images).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
There were no funding sources relevant to this case report.
Author Contributions
Catarina Gouveia: acquisition and interpretation of clinical data for and drafting of the case report; Catarina Fidalgo and Marília Cravo: conception and design of the case; critically revision of the report; and final approval of the version to be published. Rui Loureiro, Helena Oliveira, and Rui Maio: critical revision of the report and final approval of the version to be published.
References
- 1.Handra-Luca A, Terris B, Couvelard A, Bonte H, Flejou JF. Adenomyoma and adenomyomatous hyperplasia of the Vaterian system: clinical, pathological, and new immunohistochemical features of 13 cases. Mod Pathol. 2003 Jun;16((6)):530–6. doi: 10.1097/01.MP.0000073525.71096.8F. [DOI] [PubMed] [Google Scholar]
- 2.Nishimura A, Shirai Y, Hatakeyama K. Segmental adenomyomatosis of the gallbladder predisposes to cholecystolithiasis. J Hepatobiliary Pancreat Surg. 2004;11((5)):342–7. doi: 10.1007/s00534-004-0911-x. [DOI] [PubMed] [Google Scholar]
- 3.Bricker DL, Halpert B. Adenomyoma of the gallbladder. Surgery. 1963 May;53:615–20. [PubMed] [Google Scholar]
- 4.Albores-Saavedra J, Henson DE, Sobin LH. The WHO Histological Classification of Tumors of the Gallbladder and Extrahepatic Bile Ducts. A commentary on the second edition. Cancer J. 1992;70:410–414. doi: 10.1002/1097-0142(19920715)70:2<410::aid-cncr2820700207>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 5.Baggenstoss AH. Major duodenal papilla: variations of pathologic interest and lesions of the mucosa. Arch Pathol (Chic) 1938;26:853–68. [Google Scholar]
- 6.Dardinski VJ. Inflammatory adenomatoid hyperplasia of the major duodenal papilla in man. Am J Pathol. 1931 Sep;7((5)):519–522.1. [PMC free article] [PubMed] [Google Scholar]
- 7.Menzel J, Poremba C, Dietl KH, Böcker W, Domschke W. Tumors of the papilla of Vater—inadequate diagnostic impact of endoscopic forceps biopsies taken prior to and following sphincterotomy. Ann Oncol. 1999 Oct;10((10)):1227–31. doi: 10.1023/a:1008368807817. [DOI] [PubMed] [Google Scholar]
- 8.Hammarström LE, Holmin T, Stenram U. Adenomyoma of the ampulla of Vater: an uncommon cause of bile duct obstruction. Surg Laparosc Endosc. 1997 Oct;7((5)):388–93. [PubMed] [Google Scholar]
- 9.Navaneethan U, Njei B, Lourdusamy V, Konjeti R, Vargo JJ, Parsi MA. Comparative effectiveness of biliary brush cytology and intraductal biopsy for detection of malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc. 2015 Jan;81((1)):168–76. doi: 10.1016/j.gie.2014.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim JY, Choi JH, Kim JH, Kim CL, Bae SH, Choi YK, et al. [Clinical usefulness of bile cytology obtained from biliary drainage tube for diagnosing cholangiocarcinoma] Korean J Gastroenterol. 2014 Feb;63((2)):107–13. doi: 10.4166/kjg.2014.63.2.107. [DOI] [PubMed] [Google Scholar]
- 11.Uchida N, Kamada H, Ono M, Aritomo Y, Masaki T, Nakatsu T, et al. How many cytological examinations should be performed for the diagnosis of malignant biliary stricture via an endoscopic nasobiliary drainage tube? J Gastroenterol Hepatol. 2008 Oct;23((10)):1501–4. doi: 10.1111/j.1440-1746.2007.05214.x. [DOI] [PubMed] [Google Scholar]
- 12.Logrono R, Kurtycz DF, Molina CP, Trivedi VA, Wong JY, Block KP. Analysis of false-negative diagnoses on endoscopic brush cytology of biliary and pancreatic duct strictures: the experience at 2 university hospitals. Arch Pathol Lab Med. 2000 Mar;124((3)):387–92. doi: 10.5858/2000-124-0387-AOFNDO. [DOI] [PubMed] [Google Scholar]
- 13.Navaneethan U, Njei B, Venkatesh PG, Lourdusamy V, Sanaka MR. Endoscopic ultrasound in the diagnosis of cholangiocarcinoma as the etiology of biliary strictures: a systematic review and meta-analysis. Gastroenterol Rep (Oxf) 2015 Aug;3((3)):209–15. doi: 10.1093/gastro/gou057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogura T, Hara K, Hijioka S, Mizuno N, Imaoka H, Niwa Y, et al. Can endoscopic ultrasound-guided fine needle aspiration offer clinical benefit for tumors of the ampulla of vater? -an initial study. Endosc Ultrasound. 2012 Jul;1((2)):84–9. doi: 10.7178/eus.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jang KT, Heo JS, Choi SH, Choi D, Lim JH, Oh YL, et al. Adenomyoma of Ampulla of Vater or the Common Bile Duct - A Report of Three Cases. Korean J Pathol. 2005;39:59–62. [Google Scholar]
- 16.Aoun N, Zafatayeff S, Smayra T, Haddad-Zebouni S, Tohmé C, Ghossain M. Adenomyoma of the ampullary region: imaging findings in four patients. Abdom Imaging. 2005 Jan-Feb;30((1)):86–9. doi: 10.1007/s00261-004-0224-1. [DOI] [PubMed] [Google Scholar]
- 17.Martin ED, Bedossa P, Oudinot P. Les lésions de la région oddienne: fréquence et association à des lésions biliares et pancréatiques dans une série de 109 autopsies. Gastroenterol Clin Biol. 1987 Aug-Sep;11((8-9)):574–80. [PubMed] [Google Scholar]
- 18.Fernandez-Cruz L, Pera C. Proceedings of the 3rd Gastroenterology Symposium. Nice, France: 1976. Jun, A histological study of the sphincter of Oddi; pp. 8–9. [DOI] [Google Scholar]
- 19.Narita T, Yokoyama M. Adenomyomatous hyperplasia of the papilla of Vater: A sequela of chronic papillitis? Ann Diagn Pathol. 1999 Jun;3((3)):174–7. doi: 10.1016/s1092-9134(99)80045-8. [DOI] [PubMed] [Google Scholar]
- 20.Ulich TR, Kollin M, Simmons GE, Wilczynski SP, Waxman K. Adenomyoma of the papilla of Vater. Arch Pathol Lab Med. 1987 Apr;111((4)):388–90. [PubMed] [Google Scholar]
- 21.Ikei S, Mori K, Yamane T, Katafuchi S, Hirota M, Akagi M. Adenofibromyomatous hyperplasia of the extrahepatic bile duct—a report of two cases. Jpn J Surg. 1989 Sep;19((5)):576–82. doi: 10.1007/BF02471666. [DOI] [PubMed] [Google Scholar]
- 22.Legakis NC, Stamatiadis AP, Papadimitriou-Karapanou C, Apostolidis NS. Adenomyoma of the common bile duct. Arch Surg. 1990 Apr;125((4)):543. doi: 10.1001/archsurg.1990.01410160131025. [DOI] [PubMed] [Google Scholar]
- 23.Läuffer JM, Baer HU, Maurer CA, Fröhling S, Scheurer U, Zimmermann A, et al. Adenomyoma of the distal common bile duct mimicking cholangiocarcinoma. Dig Dis Sci. 1998 Jun;43((6)):1200–4. doi: 10.1023/a:1018843421292. [DOI] [PubMed] [Google Scholar]
- 24.Kalil A, Brodt M, Mastalir E. Adenomyoma of the papilla of Vater in a young adult. Rev Col Bras Cir. 2000;27((2)):138–9. [Google Scholar]
- 25.Tsukamoto T, Kinoshita H, Hirohashi K, Kubo S, Tanaka H, Hamba H, et al. Adenomyoma of the common bile duct. Hepatogastroenterology. 1999 May-Jun;46((27)):1627–30. [PubMed] [Google Scholar]
- 26.Ojima H, Takenoshita S, Nagamachi Y. Adenomyoma of the common bile duct: report of a case. Hepatogastroenterology. 2000 Jan-Feb;47((31)):132–4. [PubMed] [Google Scholar]
- 27.Kayahara M, Ohta T, Kitagawa H, Miwa K, Urabe T, Murata T. Adenomyomatosis of the papilla of Vater: a case illustrating diagnostic difficulties. Dig Surg. 2001;18((2)):139–42. doi: 10.1159/000050115. [DOI] [PubMed] [Google Scholar]
- 28.Bedirli A, Patiroglu TE, Sozuer EM, Sakrak O. Periampullary adenomyoma: report of two cases. Surg Today. 2002;32((11)):1016–8. doi: 10.1007/s005950200205. [DOI] [PubMed] [Google Scholar]
- 29.Martínez Vieira A, Durán Ferreras I, Gómez Bravo MA, Tamayo López MJ, García González I, Serrano Díez-Canedo J, et al. Ictericia obstructiva en varón de 22 años. Rev Esp Enferm Dig. 2005 Jun;97((6)):460–1. doi: 10.4321/s1130-01082005000600011. [DOI] [PubMed] [Google Scholar]
- 30.Masoom S, Venkataraman G, Hammadeh R. Symptomatic adenomyoma of the Vaterian system: a pathologic curiosity with a potential for misdiagnosis. APMIS. 2006 Jul-Aug;114((7-8)):559–61. doi: 10.1111/j.1600-0463.2006.apm_476.x. [DOI] [PubMed] [Google Scholar]
- 31.Kwon TH, Park DH, Shim KY, Cho HD, Park JH, Lee SH, et al. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol. 2007 May;13((20)):2892–4. doi: 10.3748/wjg.v13.i20.2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shu GM, Wang YJ, Du Z, Li DY, Liu CL. Bile tract adenomyoma: a case report. World J Gastroenterol. 2008 Jan;14((4)):647–50. doi: 10.3748/wjg.14.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iwaki K, Shibata K, Ohta M, Endo Y, Uchida H, Tominaga M, et al. Adenomyomatous hyperplasia of the common bile duct: report of a case. Surg Today. 2008;38((1)):85–9. doi: 10.1007/s00595-007-3558-9. [DOI] [PubMed] [Google Scholar]
- 34.Genevay M, Frossard JL, Huber O, Rubbia-Brandt L, Dumonceau JM. High-grade common bile duct stricture caused by diffuse adenomyomatosis. Gastrointest Endosc. 2009 May;69((6)):1167–8. doi: 10.1016/j.gie.2008.11.049. [DOI] [PubMed] [Google Scholar]
- 35.Lehwald N, Cupisti K, Baldus SE, Kröpil P, Schulte Am Esch J, 2nd, Eisenberger CF, et al. Unusual histological findings after partial pancreaticoduodenectomy including benign multicystic mesothelioma, adenomyoma of the ampulla of Vater, and undifferentiated carcinoma, sarcomatoid variant: a case series. J Med Case Reports. 2010 Dec;4((1)):402. doi: 10.1186/1752-1947-4-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Higashi M, Goto M, Saitou M, Shimizu T, Rousseau K, Batra SK, et al. Immunohistochemical study of mucin expression in periampullary adenomyoma. J Hepatobiliary Pancreat Sci. 2010 May;17((3)):275–83. doi: 10.1007/s00534-009-0176-5. [DOI] [PubMed] [Google Scholar]
- 37.Kumari N, Vij M. Adenomyoma of ampulla: a rare cause of obstructive jaundice. J Surg Case Rep. 2011 Aug;2011((8)):6. doi: 10.1093/jscr/2011.8.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Choi YH, Kim MJ, Han JH, Yoon SM, Chae HB, Youn SJ, et al. Clinical, pathological, and immunohistochemical features of adenomyoma in the ampulla of vater. Korean J Gastroenterol. 2013 Dec;62((6)):352–8. doi: 10.4166/kjg.2013.62.6.352. [DOI] [PubMed] [Google Scholar]
- 39.Rafiullah TS, Tanimu S. Adenomyomatous hyperplasia of the ampulla of Vater presenting as acute pancreatitis. BMJ Case Rep. 2014 Mar;2014(mar06 1):bcr2013203151. doi: 10.1136/bcr-2013-203151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Choi JH, Lee SH, Kim JS, Kim J, Shin BS, Jang DK, et al. A case of adenomyomatous hyperplasia of the distal common bile duct mimicking malignant stricture. Korean J Gastroenterol. 2016 Jun;67((6)):332–6. doi: 10.4166/kjg.2016.67.6.332. [DOI] [PubMed] [Google Scholar]
- 41.D'Assuncao MA, Armellini ST, Moribe D, Nova da Costa LS, Leite GF, Vendrame LM, et al. Adenomyoma of the common bile duct: a rare lesion diagnosed and treated by ERCP. Endoscopy. 2016;48((S 01 Suppl 1)):E266–7. doi: 10.1055/s-0042-111312. [DOI] [PubMed] [Google Scholar]
- 42.Keegan M, Karim R, Kaffes A, Saxena P. A welcome diagnosis for painless biliary dilatation (with video) Gastrointest Endosc. 2017 Sep;86((3)):568–9. doi: 10.1016/j.gie.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 43.Gialamas E, Mormont M, Bagetakos I, Frossard JL, Morel P, Puppa G. Combination of adenomyoma and adenomyomatous hyperplasia of the ampullary system: a first case report. Int J Surg Pathol. 2018 Oct;26((7)):644–8. doi: 10.1177/1066896918767561. [DOI] [PubMed] [Google Scholar]