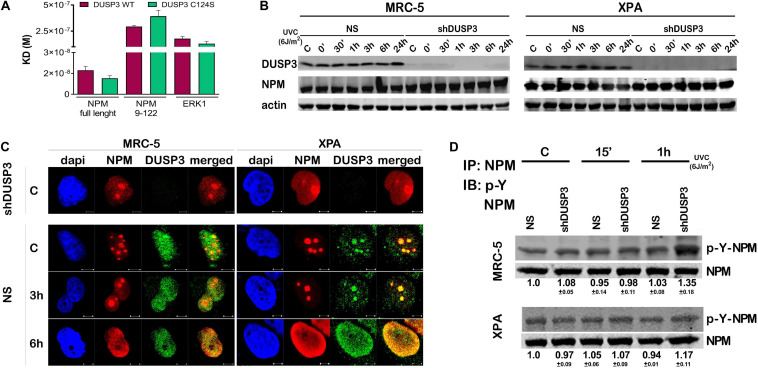
FIGURE 1.
Dual specificity phosphatase 3 (DUSP3) binds with high affinity to nucleophosmin (NPM) in vitro and affects its nuclear localization and tyrosine phosphorylation. (A) The bimolecular interaction of purified recombinant proteins was performed by Surface Plasmon Resonance (SPR) and the obtained KD# is shown in the graph. DUSP3 interacts with higher affinity to NPM full length compared to truncated NPM9–122, while ERK1 was used as classic control of DUSP3 substrate. (B) MRC-5 and XPA cell lines exposed to UVC radiation show NPM expression was not affected at all indicated times, regardless of the DUSP3 presence. (C) DUSP3 colocalizes with NPM before and after exposure to 18 J/m2 UVC. This colocalization occurs even after nucleoplasmic translocation of the NPM (complete kinetics is shown in Supplementary Figure 1). There is no signal of DUSP3 staining in the shDUSP3 cells by immunofluorescence. Representative images are only qualitative and white scale bars are 5 μM length at 63 × magnification. (D) Immunoprecipitation assays using NPM antibody and immunoblotted with anti phospho-Tyr antibody show an increase in the levels of phospho-Tyr-NPM in both MRC-5 and XPA shDUSP3 cells 1 h after UVC exposure. Immunoblottings are representative of experiments performed in triplicates, and the quantification is shown below each band as average ± standard deviation. # Note KD = ka/kd (M), where KD = equilibrium dissociation constant, ka = association rate constant, and kd = dissociation rate constant.