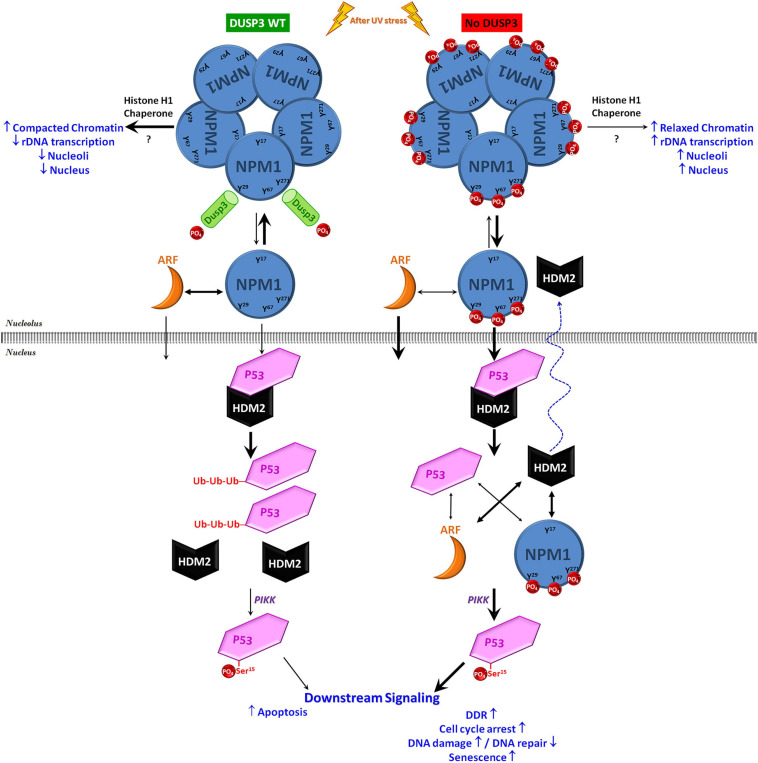
FIGURE 6.
Schematic model on the contribution of DUSP3-NPM axis to p53 actions in the maintenance of genomic stability. Under conditions of cellular homeostasis, the NPM present in the nucleoli is constantly interacting with and being dephosphorylated by DUSP3 on residues Y29, Y67, and Y271. In the absence of DUSP3, these three residues remain phosphorylated and favor the dissociation equilibrium of NPM homo-oligomerization and/or its association with ARF, therefore promoting an early nucleoplasmic translocation of monomeric NPM and ARF. Once in the nucleoplasm, these two proteins can induce the dissociation of the HDM2-p53 interaction through the individual binding to one or the other protein. This mitigates the process of p53 degradation (via proteasome), increasing its half-life and, therefore, allowing its phosphorylation in Ser15 (through kinases of the PIKK family) that subsequently increase its transcriptional activity. Therefore, as previously reported in a DUSP3 deficiency scenario, the greater p53 activation modulates the downstream pathway to regulate cellular responses to genotoxic stress, causing cell cycle arrest associated with the absence or insufficient DNA repair, followed by senescence and reduced cell proliferation/survival (Torres et al., 2017; Monteiro et al., 2019; Russo et al., 2020).