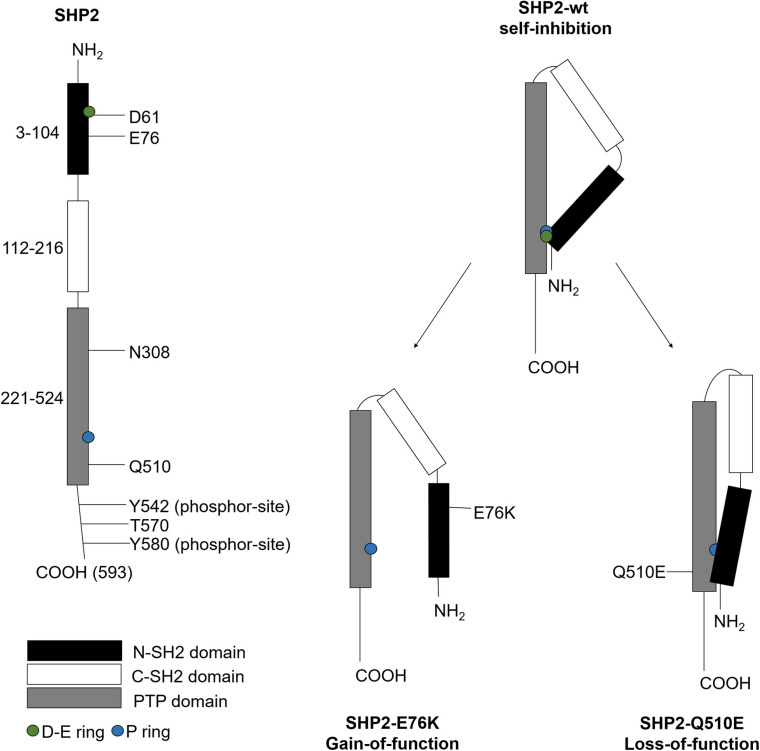
FIGURE 1.
The structure and function regulation of SHP2. SHP2 contains 593 amino acids, including various important active regulatory sites. When these sites are mutated, SHP2 gains or loses function. The D-E ring and P ring on the three-dimensional structure of SHP2 play an important role in regulating catalytic activity. When SHP2 is in self-inhibition state, the P ring on the PTP domain will cover the D-E ring on the N-SH2 domain. SHP2 is activated by gain-of-function mutations, such as E76K, or inactivated by loss-of-function mutations, such as Q510E.