Figure 3. Model of RGS12 action in the differential behavioral outputs of kappa opioid receptor (KOR) signaling, as derived from recent published work using Rgs12-deficient mouse strains.
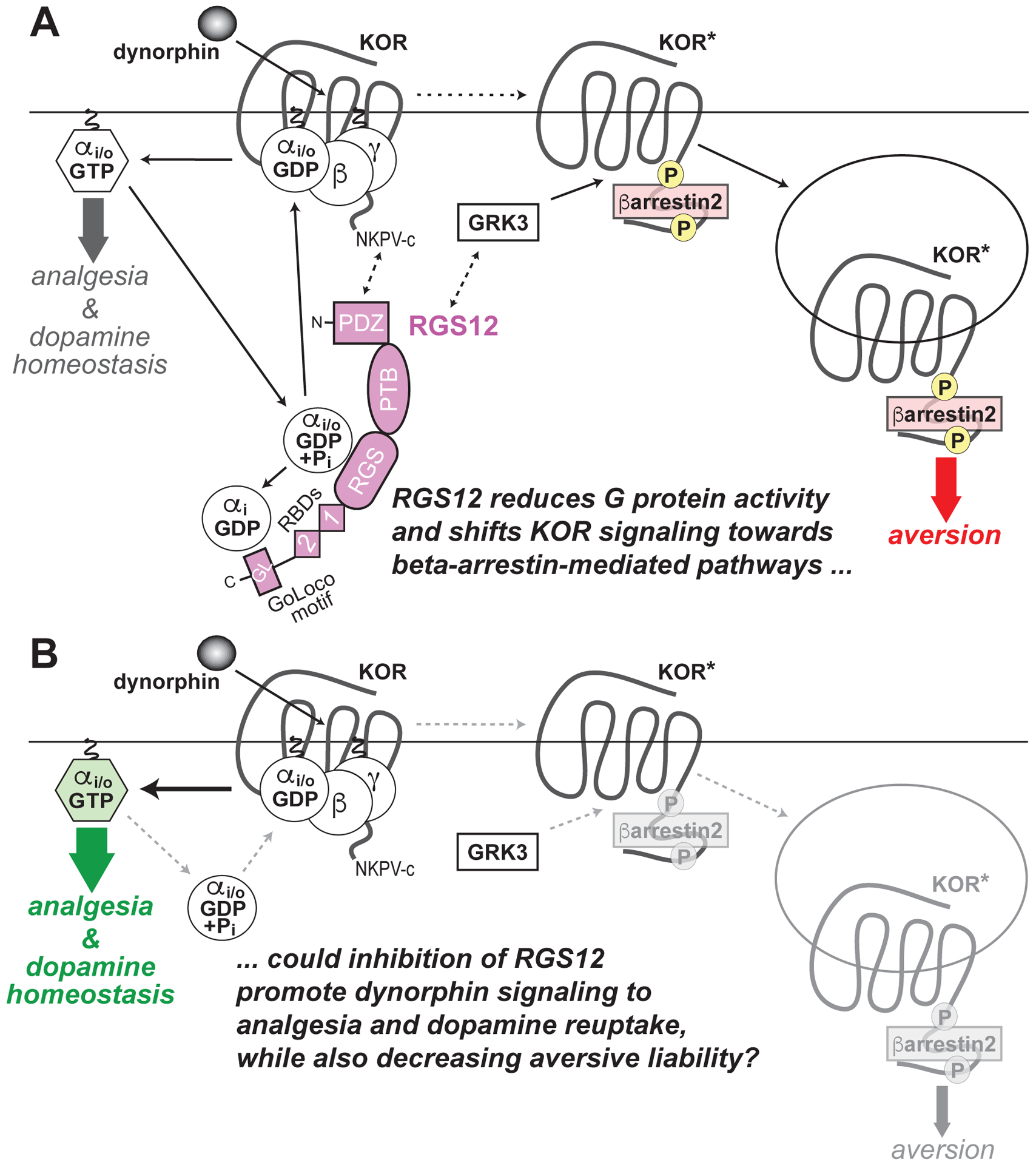
137,138 (A) Dynorphin-induced activation of KOR, as occurs during stress, dissociates Gi/o-coupled heterotrimers into free G protein subunits, evokes G protein-dependent signal transduction (thick grey arrow) and, ultimately, produces analgesia and also regulates dopamine homeostasis by the pre-synaptic dopamine transporter (DAT). RGS12 expression is known to reduce KOR-mediated G protein signaling via its central RGS domain (by accelerating GTP hydrolysis of Gαi/o·GTP) and its C–terminal GoLoco motif (by trapping Gαi·GDP). RGS12 also contains an N-terminal PDZ domain that likely interacts with the C-terminal PDZ domain docking site of KOR (‘NKPV-c’). RGS12 also contains tandem Ras-binding domains (RBDs) that we previously established interact with activated H-Ras and Raf to enable MAPK/ERK scaffolding properties150. Agonist-stimulated KOR activation also drives GRK3-dependent βarrestin2 recruitment, resulting in receptor internalization, βarrestin-dependent signal transduction (thick red arrow) and, ultimately, aversive behavior (in parallel to decreased extracellular dopamine). RGS12 expression augments KOR agonist-stimulated βarrestin recruitment independent of its GAP and GDI activity137, suggesting that RGS12 modulates GRK3 activity on activated KOR (KOR*) and/or enhances βarrestin2 recruitment and/or function by as yet undetermined molecular mechanisms. (B) Loss of RGS12 in mice results in concomitant increases in KOR-mediated G protein signaling and decreases in βarrestin2 recruitment, thus resulting in enhanced KOR agonist-stimulated analgesia and enhanced DAT function causing enhanced dopamine reuptake (thicker green arrow), as well as an attenuation of KOR-stimulated aversion (thinner grey arrow), respectively.
