FIGURE 1. Gut microbiota-derived short chain fatty acids modulate host health with implications in obesity and hypertension.
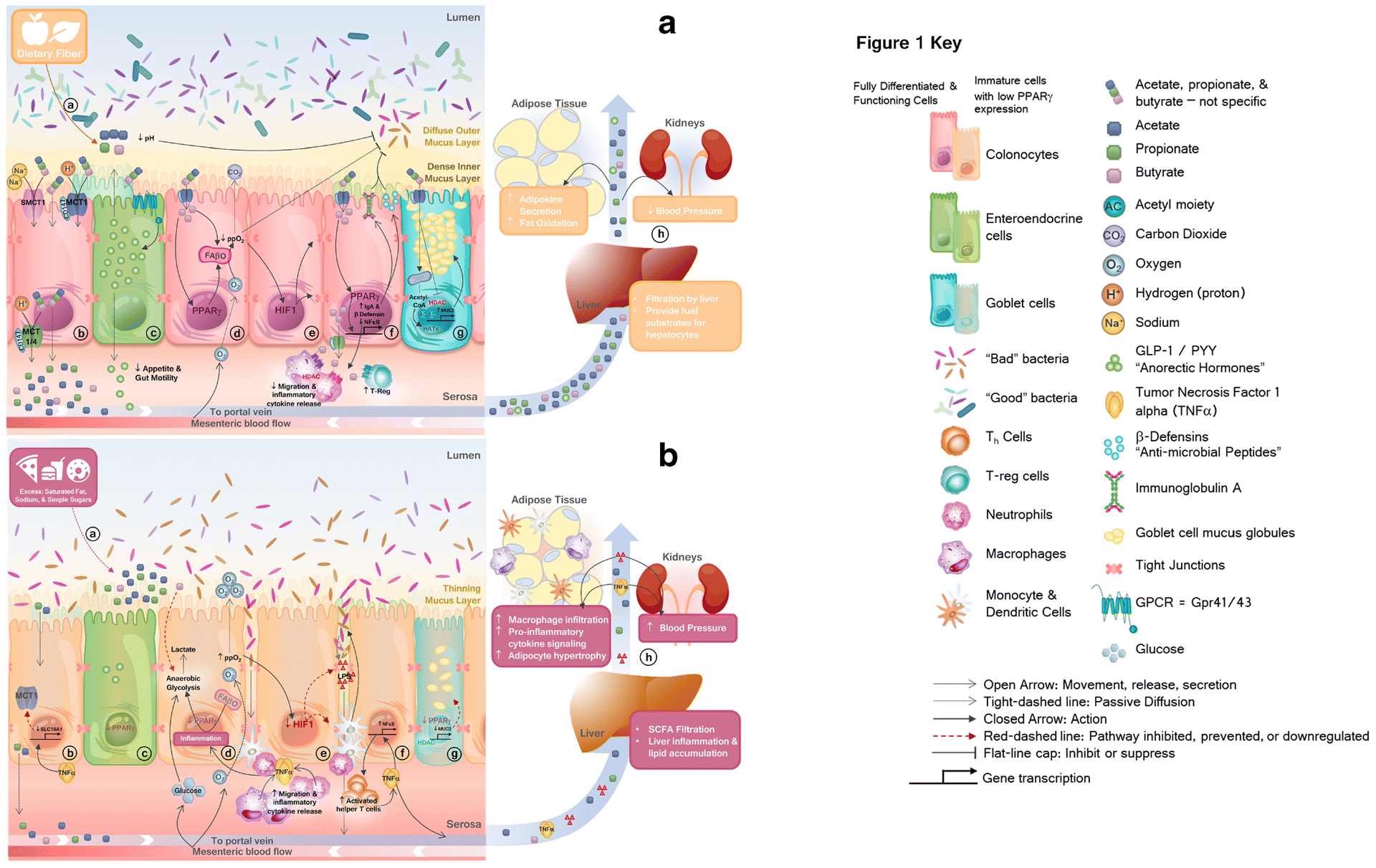
A.
a) Dietary fibers provide substrate for commensal bacteria to produce short chain fatty acids (SCFAs), acetate, propionate, and butyrate. Bacterial fermentation of dietary fibers reduces luminal pH. b) Protonated-SCFAs can passively diffuse through the membrane, while anionic-SCFAs require carrier mediated transport (by MCT1, MCT4, SMCT1 and SMCT2) c) SCFAs bind to and activate Gpr41/43, causing secretion of anorectic hormones, peptide YY (PYY) and glucagon-like peptide (GLP)-1, d) Within fully differentiated colonocytes, SCFAs activate peroxisome proliferator activated receptor gamma (PPARγ) which activates mitochondrial fatty acid beta-oxidation (FAβO), reducing luminal availability of oxygen. e) Within colonocytes, hypoxic conditions activate hypoxia-inducible factor 1 (HIF1), which upregulates expression and function of tight junction proteins, maintaining gut barrier integrity. f) Butyrate activation of PPARγ upregulates anti-inflammatory immunoglobulin A (IgA) and beta-defensin (β-defensin) expression and inhibits nuclear factor kappa b (NFκB) signaling. Butyrate enhances activation of regulatory T-cells and suppresses migration, activation, or release of pro-inflammatory mediators from resident immune cells via histone deacetylase (HDAC) inhibition and GPCR-signaling. g) Fully differentiated and functional goblet cells with sufficient PPARγ expression allows butyrate-mediated histone acetyltransferase (HAT) or HDAC-inhibition-mediated upregulation of mucin, or MUC2, maintaining a healthy mucus layer. h) SCFAs which are not used in colonocyte metabolic processes, are sent to the periphery via portal circulation where they elicit beneficial systemic effects.
B.
a) Excess dietary saturated fat, sodium, and simple sugars provide nutrient advantage for facultative anaerobic bacteria. b) Inflammation downregulates SCFA-transporters, reducing colonocyte uptake and systemic circulation of SCFA. c) Reduced SCFA signaling and activation of PPARγ within intestinal crypts dysregulates normal growth and proper differentiation of cells. d) Diminished abundance and uptake of SCFAs limit substrate available to fuel colonocytes, and may prompt a metabolic switch from high oxygen-consuming mitochondrial FAβO to anaerobic glycolysis, forcing cells to pull exogenous carbon-sources from blood-supply (i.e. glucose) resulting in lactate production. e) Loss of cellular and luminal physiological hypoxia reduces HIF1 expression, resulting in loss of tight junction and adhesion molecule expression and function. This allows bacterial translocation, triggering the innate immune response. f) Release of endotoxin lipopolysaccharide (LPS) induces pro-inflammatory NFκB signaling. g) Immature goblet cells lacking proper PPARγ and SCFA-signaling produce less mucin, leading to a reduced mucus layer. Nutrient selection, reduced bacterial competition, and increased luminal oxygen availability provide a selective advantage for pathogenic overgrowth. h) Local intestinal inflammation leads to organ-specific and systemic inflammation, increasing cardiometabolic risk.
