Abstract
Introduction:
Current American Urological Association (AUA) Best Practice Statement recommends antibiotic prophylaxis for cystoscopy with manipulation, including stent removal; although no Level 1b trials explicitly address prophylaxis for stent removal. We sought to determine the efficacy of prophylactic antibiotics to prevent infectious complications after stent removal.
Materials and Methods:
Following institutional review board approval, patients undergoing removal of ureteral stent placed during stone surgery were recruited from July 2016 to March 2019. Patients were recruited at the time of stent removal and randomized to treatment (single dose 500 mg oral ciprofloxacin) or control group (no antibiotics). Telephone contact was attempted within 14 days of stent removal to assess for urinary tract infection (UTI) symptoms, antibiotic prescriptions, or Emergency Department visits. Primary outcome was UTI within 1 month of stent removal – defined by irritative voiding symptoms, fever or abdominal pain associated with positive urine culture (Ucx) (>100k colony-forming units/mL).
Results:
Seventy-seven patients were enrolled, with 58 meeting final inclusion criteria for the analysis (33 treatment, 25 controls). No differences were seen with clinical and demographic variables, except a higher body mass index in the treatment group (P = 0.007). Positive Ucx rate before stone surgery (16.7% vs. 11.8%, P = 0.819) and at the time of stent removal (16.0% vs. 11.1%, P = 0.648) was not significantly different in treatment versus control groups, respectively. Primary outcome: No patients in either cohort developed symptomatic culture-diagnosed UTI within 1 month of stent removal. Of patients with documented phone follow-up (treatment n = 29, control n = 22), only one patient (control) reported any positive response on phone survey.
Conclusions:
We found a low infectious complication rate regardless of antibiotic prophylaxis use during cystoscopic stent removal. The necessity of antibiotics during routine cystoscopic stent removal warrants possible reevaluation of the AUA best practice statement.
Keywords: Antibiotic, cystoscopy, prophylaxis, randomized controlled trial, ureteral stent
INTRODUCTION
The current prevalence of antibiotic-resistant bacteria compels the judicious use of antibiotics across all fields of medicine. However, the specific morbidity associated with healthcare-acquired infections and surgical site infections[1] also necessitates appropriate antimicrobial prophylaxis for certain procedures. Ideally, factors such as infectious complication rates, adverse medication effects, and the cost of managing infectious complications are weighed to determine which prophylactic antibiotics, if any, will be offered. To address these considerations within urology, the American Urological Association (AUA) Best Practice Policy Statement on Urologic Surgery Antimicrobial Prophylaxis was released in 2008 and continues to be updated with the current literature.[2]
One situation with current practice variation between individual physicians is prophylaxis at the time of routine ureteral stent removal. Indwelling ureteral stents are frequently placed to ensure adequate drainage following surgical treatment of nephrolithiasis and in the absence of external strings, are generally removed cystoscopically within a few weeks of stent placement. The current AUA Best Practice recommendations for patients with normal risk profile is to forgo antibiotic prophylaxis with cystoscopy alone. However, patients undergoing cystourethroscopy with manipulation are recommended to receive <24 h of antibiotic prophylaxis.[2] This recommendation is primarily based on meta-analyses assessing the efficacy of antibiotic prophylaxis in transurethral resection of the prostate (TURP) which were subsequently generalized for all cystourethescopic procedures with manipulation.[2,3,4]
Per best policy statements, the manipulation includes transurethral resection of prostate or bladder tumor; any biopsy, resection, or fulguration; urethral dilation or urethrotomy; foreign body removal; and ureteral stent placement or removal. When comparing the minimally disruptive nature of stent removal to more invasive cystourethroscopic procedures, the postprocedural infectious risk profiles would seem intuitive to differ. To date, no level 1b trials have been performed which explicitly assess the impact of antibiotic prophylaxis for stent removal. This research group previously performed a retrospective analysis of seventy patients at UC San Diego (UCSD) undergoing cystoscopic ureteral stent removal. No significant difference was found in the infectious complication rate between those who received prophylactic antibiotics (n = 35) and those who did not (n = 35).[5] The current study is intended to extend the previous analysis using a prospective randomized controlled format. We hypothesized that no difference exists in the infectious complication rate between patients who receive antimicrobial prophylaxis at the time of cystoscopic ureteral stent removal and those who do not.
MATERIALS AND METHODS
Trial design
A randomized, prospectively controlled, clinical trial registered with ClinicalTrials.gov National Clinical Trials (NCT) 2944825 was conducted at (omittedA) and at partner practice (omittedB) following attainment of appropriate institutional review board (IRB) approval ((omitted A) IRB#160160). The Consolidated Standards of Reporting Trials were followed. Patients were enrolled during the month from July 2016 to March of 2019 with 1 month of observation after enrollment. Patients were randomized at enrollment following a parallel design with an anticipated 1:1 ratio to either treatment (<24 h single-dose oral prophylactic antibiotic) or control arm (no prophylaxis) based on a predetermined, computer-generated random allocation sequence. Randomization was performed using varied block sizes for further concealment. There was no stratification of randomization for any clinical or patient characteristics. Attending physicians directly involved in urologic care of patients were blinded to treatment allocation before and following enrollment. Clinical research staff were responsible for the randomization and maintenance of treatment allocation records. The allocation sequence was stored inside sequentially numbered sealed envelopes which were opened following enrollment. Enrollment was performed by treating physicians and by clinical research staff in both (omitted A) and (omitted B) urology clinics at the time of office flexible cystoscopy for ureteral stent removal. The target sample size was set at a minimum of 320 patients (160/site cohort) calculated with the assumption of β = 0.20, α = 0.05, with the power to detect a 6% difference in the primary outcome. Initial calculations for the intended sample size were based on the infection rates noted in the previous study by Abbott et al. with 6% observed in the antibiotic group and 0% in the prophylaxis-free cohort.[5] The results reported here represent an interval analysis given the exceedingly low observed infection rate as the trial proceeded. With a recalculation performed for the observed outcome difference of <1%, the sample size required to detect a difference would be minimum of 5600 patients.
Participants
The initial study protocol included patients 18 years of age and older of any sex or ethnicity undergoing cystoscopic ureteral stent removal within 14 days of operative stent placement. To improve the enrollment rate, this timeframe was later amended with IRB approval to include stent removal within 21 days of placement. All patients had stents placed intraoperatively while undergoing a stone treatment surgery including shockwave lithotripsy, percutaneous nephrolithotomy (PCNL), or retrograde ureteroscopy (URS). Stents were unilateral or bilateral indwelling J or Double J stents without externalized strings. Patients who were pregnant (tested before initial stone treatment surgery), patients performing clean intermittent catheterization, or patients with indwelling urethral catheters, suprapubic catheter or nephrostomy tubes were excluded from the study.
Intervention
As per the current AUA best practice policy statement, a fluoroquinolone antibiotic (ciprofloxacin) or trimethoprim-sulfamethoxazole (TMP-SMX) was utilized in the treatment arm.[2] The first choice of prophylaxis entailed <24 h of antibiotics consisting of a single oral dose of 500 mg Ciprofloxacin at stent removal. In patients with allergic or other contra-indications, a single oral dose of 160/800 mg TMP-SMX was administered. The control arm did not receive prophylactic antibiotics at the time of stent removal. No placebo was otherwise administered. Cystoscopy and stent removal was performed in standard fashion, without deviation from the standard of care. Patients who ultimately developed infectious symptoms or complications following stent removal were treated empirically based on the preference of the treating physician and followed by culture-specific antibiotics.
Outcomes
The primary outcome was urinary tract infection (UTI) development within 1 month of stent removal. UTI was defined in accordance with the current AUA published guidelines with typical symptoms (dysuria, urgency, frequency, hematuria, and/or foul-smelling urine) being suggestive, but diagnosis requiring a clean-catch midstream culture with >100k bacterial colonies/mL or catheterized urine with >10k bacterial colonies/mL.[6,7] Confirmed presence of Gram-negative sepsis was also considered to be diagnostic of a complex UTI. The secondary outcome was any reported positive symptoms on phone interviews.
Data collection
Data collected preprocedurally included demographic information, medical comorbidities, and urological history, including preoperative urine culture (Ucx) results. Ucx and post-void residual (PVR) urine volume bladder scan (PVR) was collected at the time of stent removal. Positive Ucx at the time of stent removal was only treated if the patient became symptomatic. Data collected postprocedurally included a phone call between 7 and 14 days following stent removal to assess for symptoms of UTI. Of patients unable to be contacted by phone, follow-up was performed via chart review and at subsequent clinic encounters. Attempts were made to schedule follow up clinic visits within 8 weeks of stent removal with a renal and bladder ultrasound performed at 6 weeks, as per routine postoperative care.
Data analysis
Demographic variables were reported using mean and median for continuous variables and frequency/proportions for categorical variables. Other clinical variables reported included type of stone surgery, UTI history, immunocompromised status as well as other comorbidities, and antibiotic administration at stent removal. The primary outcome, rate of postprocedural UTI, was reported and compared between the intervention and control groups using a Chi-squared test. Clinical variables that included rate of nonseptic bacteriuria, stone-free rate, and postvoid residual bladder volume at the time of stent removal were compared using Chi-squared tests or t-test as indicated. Similarly, the patient characteristics were analyzed using either Chi-squared tests or t-tests as indicated. Two-sided P values were used with a statistical significance set at P = 0.05 for all analyses. The analysis was performed using SPSS version 25 IBM (Armonk, NY, USA) statistical software produced by IBM.
RESULTS
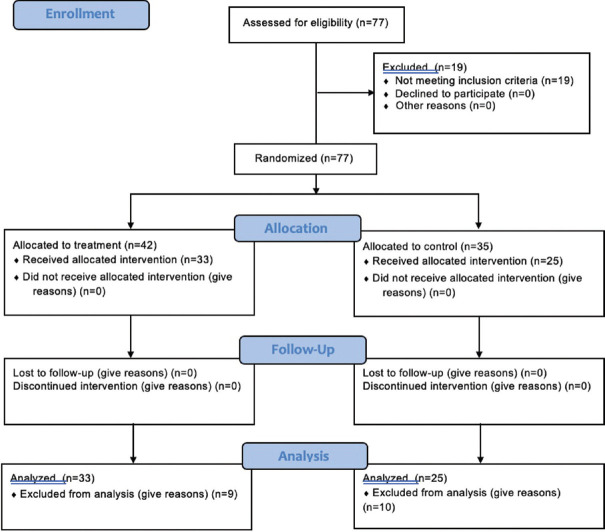
A total of 77 patients (26 [omitted B], 51 [omitted A]) were initially enrolled in the study; of these, 19 were excluded according to the initial protocol. Reasons for exclusion were stent duration >14 days (n = 15) and stent placement during a surgery other than primary stone treatment (n = 4). All 77 patients were randomized and received intended treatment; however, only 58 were included in the final analysis (22 [omitted B], 36 [omitted A]) [Figure 1]. Of these patients, 7 were unavailable for phone interview within 14 days of stent removal but were followed via chart review or clinic visit. Fifty-eight patients (33 treatment, 25 controls) underwent comparative analysis. Thirty-two of 33 treatment patients received Ciprofloxacin, and one received TMP-SMX due to allergic contraindications.
Figure 1.
Enrollment
Between the control and treatment groups, there was no significant difference in age, American Society of Anesthesiologists score, ethnicity distribution, personal history of UTI or other medical risk factors (hypertension, coronary artery disease, diabetes mellitus, chronic kidney disease, history of smoking, cancer, or immunocompromised status). Body mass index (BMI) was significantly different between groups with those patients undergoing antibiotic treatment having a higher BMI (32.3 vs. 27.1, P = 0.007) [Table 1].
Table 1.
Demographics
| Value | Combined (n=58), n (%) | Treatment (n=33), n (%) | Control (n=25), n (%) | P |
|---|---|---|---|---|
| Omitted A | 36 | 19 (53) | 17 (47) | 0.657 |
| Omitted B | 22 | 14 (64) | 8 (36) | |
| Sex (male) | 31/58 (53) | 15/33 (46) | 16/25 (64) | 0.161 |
| Ethnicity | ||||
| Asian | 3 (5) | 0 | 3 (12) | 0.099 |
| Black | 1 (2) | 1 (3) | 0 | |
| Hispanic | 11 (19) | 9 (27) | 2 (8) | |
| Other | 5 (9) | 3 (9) | 2 (8) | |
| White | 38 (66) | 20 (61) | 18 (72) | |
| Age, mean±SD | 53.4±14.2 | 50.6±13.7 | 57.2±14.3 | 0.084 |
| BMI, mean±SD | 30.1±7.4 | 32.3±8.1 | 27.1±5.0 | 0.007 |
| ASA, mean±SD | 2.4±0.6 | 2.47±0.61 | 2.37±0.62 | 0.639 |
| Hx prev UTI | 16/58 (28) | 8/33 (24) | 8/25 (32) | 0.513 |
| Hx HTN | 30/58 (52) | 17/33 (52) | 13/25 (52) | 0.971 |
| Hx CAD | 6/58 (10) | 3/33 (9) | 3/25 (12) | 0.719 |
| Hx immunocomp | 6/58 (10) | 3/33 (9) | 3/25 (12) | 0.719 |
| Hx DM | 13/58 (22) | 9/33 (27) | 4/25 (16) | 0.308 |
| Hx CKD | 5/58 (9) | 2/33 (6) | 3/25 (12) | 0.425 |
| Hx smoking | 15/58 (26) | 7/33 (21) | 8/25 (32) | 0.353 |
| Hx cancer | 7/58 (12) | 3/33 (9) | 4/25 (16) | 0.424 |
SD: Standard deviation, BMI: Body mass index, ASA: American Society of Anesthesiologist, UTI: Urinary tract infection, HTN: Hypertension, CAD: Coronary artery disease, DM: Diabetes mellitus, CKD: Chronic kidney disease
Procedural variables and stone characteristics between treatment and control groups did not vary significantly. No difference was seen between surgical procedure (URS vs. PCNL), procedure laterality, total stone burden, stone location (renal vs. ureteral vs. both), stone composition, postoperative day of stent removal, and PVR at the time of stent removal. Positive Ucx obtained preoperatively and at the time of stent removal was not significantly different between those patients who received prophylactic antibiotics and those who did not [Tables 2 and 3].
Table 2.
Procedural variables
| Value | Combined (n=58), n (%) | Treatment (n=33), n (%) | Control (n=25), n (%) | P |
|---|---|---|---|---|
| Primary procedure | ||||
| PCNL | 11/58 (19) | 4/33 (12) | 7/25 (28) | 0.127 |
| URS | 47/58 (81) | 29/33 (88) | 18/25 (72) | |
| Laterality | ||||
| Left | 29/58 (50) | 13/33 (39) | 16/25 (64) | 0.177 |
| Right | 23/58 (40) | 16/33 (49) | 7/25 (28) | |
| Bilateral | 6/58 (10) | 4/33 (12) | 2/25 (8) | |
| Total stone burden (mm), mean±SD | 12.5±9.8 | 11.1±9.2 | 14.4±10.3 | 0.207 |
| Total stone burden (mm) (median) | 10 | 10 | 15 | |
| Stone location | ||||
| Renal | 32/58 (55) | 18/33 (55) | 14/25 (56) | 0.103 |
| Ureter | 18/58 (31) | 12/33 (36) | 6/25 (24) | |
| Both | 6/58 (10) | 1/33 (3) | 5/25 (20) | |
| Stone composition (primary, if known) | ||||
| CaOx | 26/34 (77) | 17/20 (85) | 9/14 (64) | 0.465 |
| CaPhos | 4/34 (12) | 1/20 (5) | 3/14 (21) | |
| Uric acid | 2/34 (6) | 1/20 (5) | 1/14 (7) | |
| Other | 2/34 (6) | 1/20 (5) | 1/14 (7) |
PCNL: Percutaneous nephrolithotomy, URS: Ureteroscopy, SD: Standard deviation
Table 3.
Outcomes
| Value | Combined (n=58) | Treatment (n=33) | Control (n=25) | P |
|---|---|---|---|---|
| POD stent removal, mean±SD | 9.5±3.2 | 9.1±2.9 | 10.1±3.4 | 0.223 |
| PVR at removal (median) (cc) | 17 | 19 | 15 | |
| PVR at removal, mean±SD | 67.5±53.4 | 35.1±55.0 | 110.1±243.8 | 0.143 |
| Ucx preoperative (positive), n (%) | 7/48 (14.6) | 3/27 (11.1) | 4/21 (19.0) | 0.440 |
| UCx at removal (positive), n (%) | 6/54 (11.1) | 4/31 (12.9) | 2/23 (8.7) | 0.627 |
| UTI within 1 month removal | 0/58 | 0/33 | 0/25 | N/A |
| Any survey response positive | 1/51 | 1/29 | 0/22 | N/A |
N/A: Not available, PVR: Postvoid residual, UCx: Urine culture, UTI: Urinary tract infection, POD: Postoperative day
Outcomes compared between treatment and control group included UTI within 1 month of stent removal as assessed by chart review or clinic follow-up and any positive responses to phone survey questions. No patients in either the treatment or control group had a documented positive Ucx within 1 month of stent removal per AUA definition.[7] One patient reported positive responses to phone interview. The subject contacted the urologic health-care team within 2 weeks of stent removal and reported both frequency and cloudy urine. The patient ultimately had a negative Ucx result but received empiric antibiotics before the final Ucx results. This patient was part of the control group, and no other subjects reported any positive responses.
DISCUSSION
Existing literature on the necessity of antibiotic prophylaxis for ureteral stent removal is scarce, with no prospective studies yet published. The Centers for Disease Control has stated that “Antibiotic resistance is one of our most serious health threats” and states improving antibiotic stewardship is one of four core actions available to combat this threat.[8] This study represents an opportunity to identify and reduce unnecessary antibiotic use, theoretically helping to lower healthcare costs and combat antibiotic resistance. Given the absence of infectious complication in either control or prophylactic group, our prospective analysis revealed no significant differences when omitting prophylactic antibiotics for routine ureteral stent removal.
Our results indicate that between those patients who received prophylactic antibiotics and those who did not, the overall rate of infectious symptoms within the next 14 days and documented UTI within the following month was exceedingly low. No infections were recorded of 58 patients included for the analysis, and only one patient had subjective symptoms of UTI accompanied by a negative Ucx. These results support the retrospective study published by Abbott et al. demonstrating no significant difference in infectious complication rate between patients receiving either antibiotic prophylaxis or nothing at the time of cystoscopic stent removal.[5] Similarly, recent meta-analyses of antimicrobial prophylaxis for flexible cystoscopy alone have not demonstrated sufficient benefit to warrant prophylactic antibiotics at the time of cystoscopy[9,10] as reflected by the AUA guidelines.
In contrast, previous prospective studies and meta-analyses performed of TURP and transurethral resection of bladder tumor have indeed demonstrated a significantly reduced rate of postprocedural infections with prophylaxis.[3,11] The current AUA best practice guidelines on antibiotic prophylaxis for cystoscopy with stent removal relies on data generalized from TURP, a procedure which quite possibly presents an inherent difference in postprocedural infection risk.[2] Therefore, this study serves to highlight the notion that not all cystoscopic procedures will convey equivalent risk for infectious complications.
The primary limitation of this study is the reported sample size. This is particularly difficult to address given the low incidence of UTI following cystoscopy and the necessary trial size to establish a significant difference in event rate. As discussed in the methods and materials section, the sample size required to detect a difference given the currently observed infection rate (<1%) would be a minimum 5600. Given this consideration, this manuscript still represents the first randomized controlled trial specific to cystoscopic stent removal. Future trials may be structured over multiple sites to accommodate the volume of enrollment needed to appropriately power a study to detect differences in lower-than-expected infection rates noted in this study. Additionally, one might argue this trial could be strengthened with the inclusion of a placebo pill for patients in the control arm. However, the objective signs and symptoms of a UTI should in theory be less influenced by the inclusion of a placebo pill than a study reliant upon subjective values as a primary outcome. We do caution that our results should be individualized as not every risk factor was studied, for example, our results could not be generalized to a subject with postoperative infectious event. Finally, while this trial focused on ureteral stents placed following stone surgery exclusively, there are other situations which can result in stent placement. Stents placed following a pyeloplasty, for instance, may have statistically different infection rates and duration of indwelling stents. Ultimately, while these alternative reasons for stent placement may require further analysis, we would argue that routine stent removal following any type of primary stone surgery is more similar in postprocedural infectious risk to routine cystourethroscopy without manipulation than to a cystoscopic resection or fulguration procedure which benefits from prophylactic antibiotic administration.
CONCLUSIONS
This trial is the first to prospectively address whether prophylactic antibiotics affect infection rate following cystoscopic ureteral stent removal. The initial results of this study did not demonstrate a difference in infectious complications between patients who did and did not receive prophylaxis. Further enrollment may improve the statistical power of this study, but taken alone, and it is enough to cast doubt on the need for prophylactic antibiotics during routine cystoscopic stent removal.
Financial support and sponsorship
Nil.
Conflicts of interest
Roger Sur, Boston Scientific - Consultant, Speaker; Cook Medical - Speaker; Karl Storz - Speaker; Retrophin - Speaker, Advisory Board; Kalera Medical Inc - Chief Medical Officer. Seth Bechis, Intuitive Surgical - Consultant; Boston Scientific - Consultant; Karl Storz - Speaker.
Acknowledgments
All research was performed with appropriate IRB approval and in maximal attempted accordance with guidelines for conduction of randomized controlled trials.
REFERENCES
- 1.Bratzler DW, Houck PM Surgical Infection Prevention Guideline Writers Workgroup. Antimicrobial prophylaxis for surgery: An advisory statement from the National Surgical Infection Prevention Project. Am J Surg. 2005;189:395–404. doi: 10.1016/j.amjsurg.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 2.Wolf JS, Jr, Bennett CJ, Dmochowski RR, Hollenbeck BK, Pearle MS, Schaeffer AJ, et al. Best practice policy statement on urologic surgery antimicrobial prophylaxis. J Urol. 2008;179:1379–90. doi: 10.1016/j.juro.2008.01.068. [DOI] [PubMed] [Google Scholar]
- 3.Berry A, Barratt A. Prophylactic antibiotic use in transurethral prostatic resection: A meta-analysis. J Urol. 2002;167:571–7. doi: 10.1016/S0022-5347(01)69088-8. [DOI] [PubMed] [Google Scholar]
- 4.Qiang W, Jianchen W, MacDonald R, Monga M, Wilt TJ. Antibiotic prophylaxis for transurethral prostatic resection in men with preoperative urine containing less than 100,000 bacteria per ml: A systematic review. J Urol. 2005;173:1175–81. doi: 10.1097/01.ju.0000149676.15561.cb. [DOI] [PubMed] [Google Scholar]
- 5.Abbott JE, Han A, McDonald M, Lakin C, Sur RL. Are antibiotics necessary during routine cystoscopic stent removal? Transl Androl Urol. 2016;5:784–8. doi: 10.21037/tau.2016.08.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shoskes DA, Morey AF. Urinary Tract Infections Retrieved From: The American Urological Association Educational Review Manual in Urology Castle. 3rd ed. 23. New York, NY: Connelly Grad Med Publishing; 2011. pp. 737–66. [Google Scholar]
- 7.Pontari M. AUA Core Curriculum for Residents “Urinary Tract Infections [Google Scholar]
- 8.Centers for Disease Control. Antibiotic Resistance Threats in the United States Centers for Disease Control. 2013 [Google Scholar]
- 9.Garcia-Perdomo HA, Jimenez-Mejias E, Lopez-Ramos H. Efficacy of antibiotic prophylaxis in cystoscopy to prevent urinary tract infection: A systematic review and meta-analysis. Int Braz J Urol. 2015;41:412–24. doi: 10.1590/S1677-5538.IBJU.2014.0198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carey MM, Zreik A, Fenn NJ, Chlosta PL, Aboumarzouk OM. Should we use antibiotic prophylaxis for flexible cystoscopy? A systematic review and meta-analysis. Urol Int. 2015;95:249–59. doi: 10.1159/000381882. [DOI] [PubMed] [Google Scholar]
- 11.MacDermott JP, Ewing RE, Somerville JF, Gray BK. Cephradine prophylaxis in transurethral procedures for carcinoma of the bladder. Br J Urol. 1988;62:136–9. doi: 10.1111/j.1464-410x.1988.tb04292.x. [DOI] [PubMed] [Google Scholar]